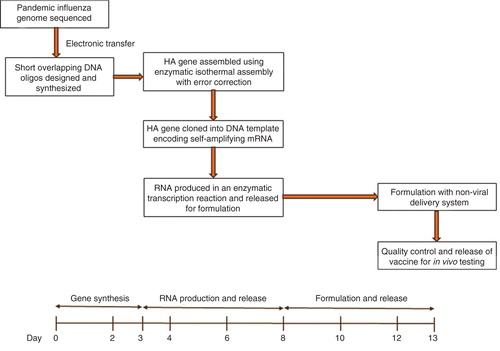
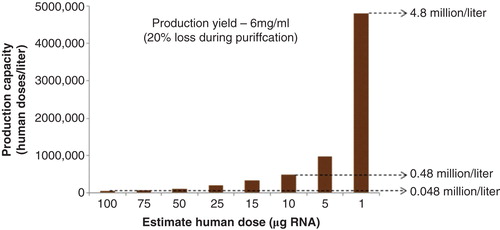
Figures & data
Table 1. Time-line of critical discoveries and developments that led to the recent progress on the non-viral delivery of self-amplifying mRNA vaccines.