Figures & data
Figure 1. Progression model of molecular carcinogenesis of oral squamous cell carcinoma.
An adapted version of the genetic progression model of head and neck squamous cell carcinoma (HNSCC).[Citation18]A genetically altered stem cell forms a ‘patch’ of clonally related daughter cells with the same genetic alteration (e.g. mutation in TP53). Such a patch can, for example, be detected by immunostaining for mutated p53. Subsequent genetic changes provide a survival benefit or enhanced proliferation for the altered stem cells and they laterally replace the normal epithelial stem cells, which results in the formation of a larger precancerous field. As the field becomes larger, additional genetic hits give rise to various subclones within the field (clonal divergence) that take over the field. Finally, a subclone acquires sufficient molecular alterations to transform into an invasive tumor cell. Three critical steps can be discriminated in this model: the first mutation causing a genetically altered patch, the outgrowth of a single mutated stem cell into a group of mutated stem cells generating the field and the transformation of a premalignant field into invasive cancer. Predictors of malignant transformation are aneuploidy and the accumulation of cancer-associated genetic changes. Well-known altered pathways in HNSCC are depicted for the three genetic subtypes that are now distinguished, that is, human papillomavirus (HPV)-positive HNSCC, HPV-negative HNSCC with few copy number alterations (low chromosomal instability (CIN)) and HPV-negative HNSCC with many copy number alterations (high CIN). HPV-negative, low CIN tumors are characterized by enrichment of HRAS activating mutations, CASP8 inactivating mutations and absence of TP53 mutations. Future studies are necessary to further characterize these tumors molecularly, particularly to determine the early steps in carcinogenesis.Moreover, many recurrent genomic alterations cannot be depicted in the progression model to date. For some alterations, the timing during carcinogenesis is known, but the involved genes and pathways are not. Examples are 3p loss (early step), 7q gain (late step) and 8p loss (late step). For other alterations, the involved genes and pathways are known, but their timing is not. Examples are inactivating mutations in FAT1, AJUBA, NOTCH1 and activation of FGFR1 (HPV-negative tumors) and FGFR3 (HPV-positive tumors).Important genetic and chromosomal alterations are indicated in the upper yellow boxes. A distinction is made between oncogenic pathways (blue boxes) and tumor-suppressive pathways (orange boxes). ↑ indicates overexpression or gain; ↑↑ indicates high-level amplification; ↓ indicates loss; and ↓↓ indicates homozygous loss. CASP8: caspase 8, apoptosis-related cysteine peptidase; CCND1: cyclin D1; CDK: cyclin-dependent kinase; CDKN2A: cyclin-dependent kinase inhibitor 2A (p16); EGFR: epidermal growth factor receptor; HRAS: Harvey rat sarcoma viral oncogene homolog; mt: mutated; PIK3CA: phosphoinositide-3 kinase subunit-α; PTEN: phosphatase and tensin homolog; TGFβ: transforming growth factor-β.Adapted from [Citation18] with permission from Nature Publishing Group.
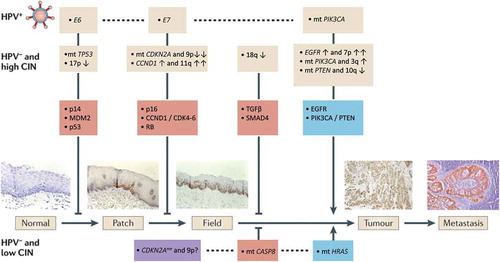
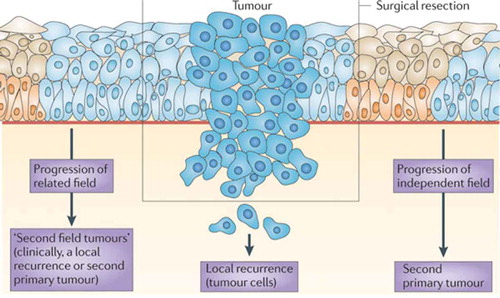
Figure 2. Field cancerization and local relapse.
Field cancerization and its role in the development of local recurrences and second primary tumors is shown.[Citation18] Premalignant fields are mucosal areas with epithelial cells that have cancer-associated genetic or epigenetic alterations. Precursor fields, which are shown in light blue, consist of cells that do not show invasive growth despite their cancer-associated genetic changes. An important clinical implication of a field is that it may be the source of local recurrences and second primary tumors after surgical resection of the initial carcinoma. This figure describes the three sources of recurrent disease: true local recurrence that derives from cancer cells that stayed behind; a second tumor from premalignant cells that stayed behind and is clonally related to the index tumor (‘second field tumor’); and a second tumor from unrelated premalignant cells (‘second primary tumor’). At present, the distinction is made on the basis of clinical criteria. Local recurrences are defined as tumors that arise within 2 cm and within 3 years of the original primary tumor. Second primary tumors are defined by any tumor that arises beyond 2 cm of the primary tumor or after 3 years at the same location.[Citation20] The distinction between local recurrences and second field tumors can only be made with molecular analysis. Index tumor, premalignant field in corresponding surgical margins and recurrent tumor should be of common clonal origin for the recurrent tumor to be considered a second field tumor. Less laborious techniques to distinguish between the two types of local recurrence would be helpful, but have not been developed at the time. The distinction does not have clinical consequences at present, but this will change when targeted chemopreventive treatment for fields becomes available. Adapted from [18] with permission from Nature Publishing Group.