Figures & data
Figure 1. Determinants of the HPV-16/18-vaccine benefit–risk with highlights of investigative strategies.
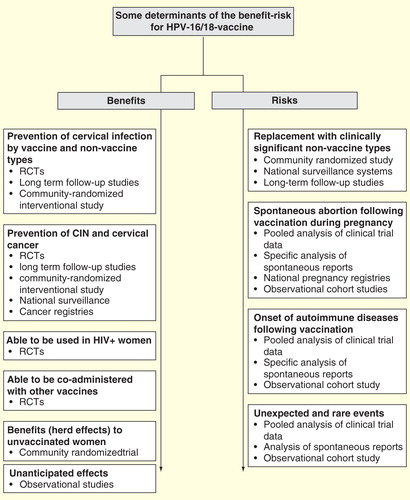
Table 1. Summary of post-licensure studies to evaluate the benefit–risk of HPV-16/18-vaccination.
Table 2. Summary of post-licensure national surveillance activities to evaluate the benefit–risk of HPV-16/18-vaccination.
