Figures & data
Table 1. Overview of studies.
Table 2. Vaccine efficacy against persistent cervical infection and ASC-US+ associated with HPV-16/18 in women with no baseline evidence of infection with the HPV type under analysis.
Table 3. Vaccine efficacy against CIN1+, CIN2+, and CIN3+ associated with HPV-16/18 in women with no baseline evidence of infection with the HPV type under analysis.
Table 4. Cross-protective vaccine efficacy against infection and CIN2+ in women with no baseline evidence of infection with the HPV type under analysis (ATP cohort).
Figure 2. Vaccine efficacy against CIN2+ and CIN3+ associated with a composite of 12 non-vaccine HPV types, with or without HPV-16/18 coinfection and excluding HPV-16/18 coinfection (PATRICIA).[Citation27] ATP: according to protocol – women DNA-negative for HPV type under analysis at baseline; CI: confidence interval; CIN: cervical intraepithelial neoplasia; HPV: human papillomavirus; PATRICIA: PApilloma TRial against Cancer in young Adults; TVC: total vaccinated cohort – women irrespective of HPV status at baseline; TVC-naïve: women DNA-negative for all HPV types tested at baseline;
![Figure 2. Vaccine efficacy against CIN2+ and CIN3+ associated with a composite of 12 non-vaccine HPV types, with or without HPV-16/18 coinfection and excluding HPV-16/18 coinfection (PATRICIA).[Citation27] ATP: according to protocol – women DNA-negative for HPV type under analysis at baseline; CI: confidence interval; CIN: cervical intraepithelial neoplasia; HPV: human papillomavirus; PATRICIA: PApilloma TRial against Cancer in young Adults; TVC: total vaccinated cohort – women irrespective of HPV status at baseline; TVC-naïve: women DNA-negative for all HPV types tested at baseline;](/cms/asset/8dbf975a-78b4-497c-8807-4aa4fe0e71b2/ierv_a_1124763_f0002_b.gif)
Figure 3. Vaccine efficacy against CIN1+, CIN2+, CIN3+, and AIS irrespective of HPV DNA (4-year, end-of-study analysis of PATRICIA).[Citation26] AIS: adenocarcinoma in situ; CI: confidence interval; CIN: cervical intraepithelial neoplasia; HPV: human papillomavirus; PATRICIA: PApilloma TRial against Cancer in young Adults; TVC: total vaccinated cohort – women irrespective of HPV status at baseline; TVC-naïve: women DNA-negative for all HPV types tested at baseline.
![Figure 3. Vaccine efficacy against CIN1+, CIN2+, CIN3+, and AIS irrespective of HPV DNA (4-year, end-of-study analysis of PATRICIA).[Citation26] AIS: adenocarcinoma in situ; CI: confidence interval; CIN: cervical intraepithelial neoplasia; HPV: human papillomavirus; PATRICIA: PApilloma TRial against Cancer in young Adults; TVC: total vaccinated cohort – women irrespective of HPV status at baseline; TVC-naïve: women DNA-negative for all HPV types tested at baseline.](/cms/asset/84d1a68e-b838-40ad-b738-976ca02a9dc2/ierv_a_1124763_f0003_b.gif)
Figure 4. Number of cases of CIN3+ associated with vaccine and non-vaccine HPV types in the TVC and TVC-naïve (4-year, end-of-study analysis of PATRICIA).[Citation26] CI: confidence interval; CIN: cervical intraepithelial neoplasia; HPV: human papillomavirus; PATRICIA: PApilloma TRial against Cancer in young Adults; TVC: total vaccinated cohort – women irrespective of HPV status at baseline; TVC-naïve: women DNA-negative for all HPV types tested at baseline. Reprinted from The Lancet Oncology, Lehtinen et al, Vol 13, Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomized, double-blind PATRICIA trial, pages 89–99, copyright 2012 with permission from Elsevier.
![Figure 4. Number of cases of CIN3+ associated with vaccine and non-vaccine HPV types in the TVC and TVC-naïve (4-year, end-of-study analysis of PATRICIA).[Citation26] CI: confidence interval; CIN: cervical intraepithelial neoplasia; HPV: human papillomavirus; PATRICIA: PApilloma TRial against Cancer in young Adults; TVC: total vaccinated cohort – women irrespective of HPV status at baseline; TVC-naïve: women DNA-negative for all HPV types tested at baseline. Reprinted from The Lancet Oncology, Lehtinen et al, Vol 13, Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomized, double-blind PATRICIA trial, pages 89–99, copyright 2012 with permission from Elsevier.](/cms/asset/6a248b9a-8b1f-4605-a474-f9c1084b08ab/ierv_a_1124763_f0004_b.gif)
Figure 5. Vaccine efficacy against CIN2+ irrespective of HPV type in the lesion versus the contribution of HPV-16/18 to the lesion: a cross-trial comparison. In the CVT, the a priori analysis included all CIN2+ cases; the exploratory analysis considered evidence of HPV persistence preceding referral to colposcopy. Cohorts considered in the analyses: ATP – in the CVT, the analysis considered outcomes that occurred in the absence of HPV during the vaccination period; TVC – in the HPV-001/007/023 study, only women who were DNA-negative for all HPV types tested were enrolled; TVC-naïve: women DNA-negative for all HPV types tested at baseline. ATP: according to protocol; CI: confidence interval; CIN: cervical intraepithelial neoplasia; CVT: Costa Rica Vaccine Trial; HPV: human papillomavirus; PATRICIA: PApilloma TRial against Cancer in young Adults; TVC: total vaccinated cohort.
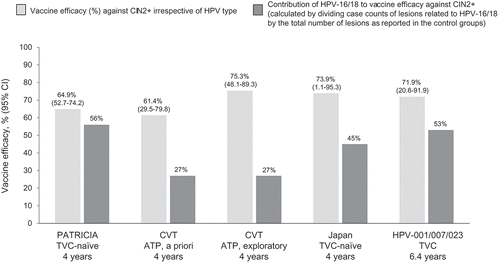
Figure 6. Vaccine efficacy against HPV-16/18 incident one-time detection and 6-month persistent infection with three, two, and one dose of HPV-16/18 vaccine (combined analysis of PATRICIA and the CVT).[Citation50] Women were excluded if they had less than 12 months of follow-up. 6MPI: 6-month persistent infection; CI: confidence interval; CVT: Costa Rica HPV-16/18 Vaccine Trial; HPV: human papillomavirus; PATRICIA: PApilloma TRial against Cancer in young Adults; m-TVC: modified total vaccinated cohort – excluding women DNA-positive at baseline for the HPV type under evaluation; TVC-naïve: women DNA-negative for all HPV types tested at baseline.
![Figure 6. Vaccine efficacy against HPV-16/18 incident one-time detection and 6-month persistent infection with three, two, and one dose of HPV-16/18 vaccine (combined analysis of PATRICIA and the CVT).[Citation50] Women were excluded if they had less than 12 months of follow-up. 6MPI: 6-month persistent infection; CI: confidence interval; CVT: Costa Rica HPV-16/18 Vaccine Trial; HPV: human papillomavirus; PATRICIA: PApilloma TRial against Cancer in young Adults; m-TVC: modified total vaccinated cohort – excluding women DNA-positive at baseline for the HPV type under evaluation; TVC-naïve: women DNA-negative for all HPV types tested at baseline.](/cms/asset/20aff9d1-d57a-40f6-9bb2-a7f1410dcc2e/ierv_a_1124763_f0006_b.gif)
Panel A. Study cohorts.
Panel B. Primary analysis populations reported for each study according to cohort and endpoint.