Figures & data
Figure 1. Overview of the manufacturing process of a biologic in a mammalian cell culture system. The manufacturing process consists of a cell culture process and a purification process. The cell culture process is initiated by expansion of a single vial of cell stock to culture flask, after which it is sequentially subcultured to larger bioreactors. In this process, optimized cell culture conditions such as temperature, agitation rate, osmolality, pH, concentration of CO2 and glucose concentration are tightly maintained, as these conditions are critical to the quality of biologics. The supernatants are harvested and further purified through several steps of chromatography, filtration and viral inactivation in the purification process, which also have potential to influence the quality of biologics.
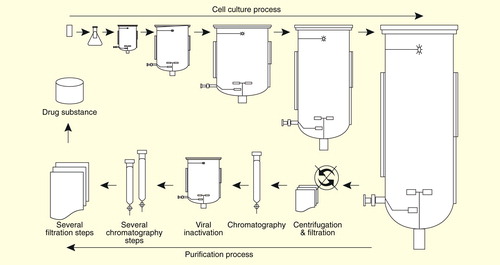
Figure 2. Comparability exercise for process change and biosimilarity. There are three types of variation: batch-to-batch, pre- and post-process-changed products, and original products and biosimilars. Appropriate comparability analyses should be performed for each case. The comparability exercise for biosimilars is more comprehensive than for process-changed products, and clinical studies are required by regulatory authorities unlike for process-changed products.
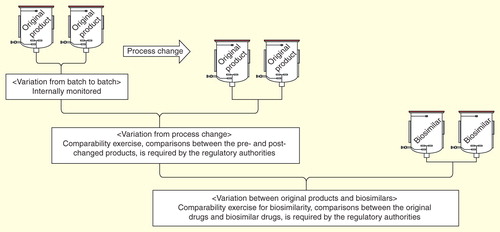
Figure 3. Mean (±SD) serum concentrations of infliximab reference medicinal product (RMP) and CT-P13 over time from PLANETAS.
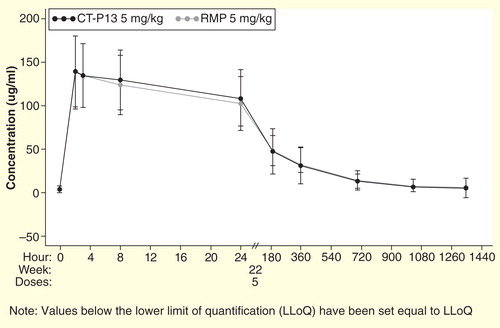
Figure 4. Impact of CT-P13 and infliximab reference medicinal product (RMP) on (A) ASAS20 response, (B) ASAS40 response, and (C) ASAS-CRP score in the PLANETAS study at weeks 14 and 30 Citation[28].
![Figure 4. Impact of CT-P13 and infliximab reference medicinal product (RMP) on (A) ASAS20 response, (B) ASAS40 response, and (C) ASAS-CRP score in the PLANETAS study at weeks 14 and 30 Citation[28].](/cms/asset/6646e0f9-126d-45ba-a8d5-27ff69d1f25d/ierm_a_1090310_f0004_b.jpg)
Table 1. Most common treatment-related TEAEs in PLANETAS and PLANETRA over 30 weeks Citation[28,29].
Figure 5. American College of Rheumatology (ACR) response rates with CT-P13 and infliximab reference medicinal product (RMP) in the PLANETRA study Citation[29]. (A) ACR20 improvement criteria at week 30 (primary efficacy end point) for the intention-to-treat (ITT) (n = 302 and 304 in the CT-P13 and RMP groups, respectively) and per-protocol (PP) populations (n = 248 and 251 patients in CT-P13 and RMP groups, respectively). (B) ACR20, ACR50 or ACR70 improvement criteria at week 14 for the PP population. (C) ACR50 or ACR70 improvement criteria at week 30 for the PP population.
![Figure 5. American College of Rheumatology (ACR) response rates with CT-P13 and infliximab reference medicinal product (RMP) in the PLANETRA study Citation[29]. (A) ACR20 improvement criteria at week 30 (primary efficacy end point) for the intention-to-treat (ITT) (n = 302 and 304 in the CT-P13 and RMP groups, respectively) and per-protocol (PP) populations (n = 248 and 251 patients in CT-P13 and RMP groups, respectively). (B) ACR20, ACR50 or ACR70 improvement criteria at week 14 for the PP population. (C) ACR50 or ACR70 improvement criteria at week 30 for the PP population.](/cms/asset/6cb2fa74-3516-4dae-9ac3-b26b01bc90ec/ierm_a_1090310_f0005_b.jpg)
