Figures & data
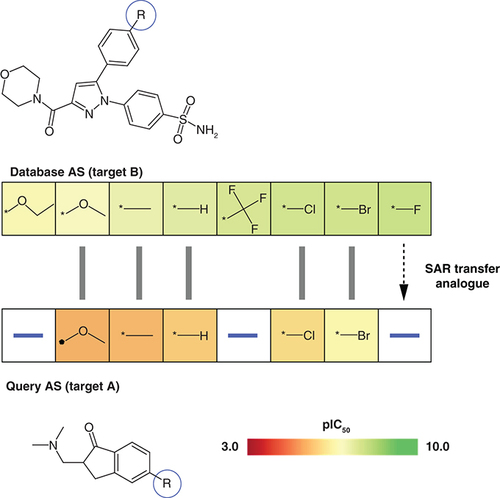
Shown is an exemplary alignment of a query AS with activity against target A (bottom) and a database AS active against another target B (top). Compounds in both ASs are arranged in the order of increasing potency. Hence, the alignment represents an SAR transfer event. For each AS, the core structure is shown and the substitution site (-R) is encircled in blue. Aligned analogues are indicated by grey bars. Cells containing R-groups of analogues are color-coded by compound potency (negative logarithmic IC50 values) according to the continuous color spectrum. The database AS contains a potent fluoro analogue (right) that is absent in the query AS. Accordingly, the fluoro derivative is suggested as an SAR transfer analogue for the query AS.
AS: Analogue series; SAR: Structure–activity relationship.