Figures & data
Figure 1 Raltegravir concentration–time curves for the adult formulation (triangles, n = 21) in P1066 cohorts I (≥12 to <19 years) and IIA (≥6 to <12 years) and the chewable tablet formulation (circles, n = 22) in cohorts ii (≥6 to <12 years) and III (≥2 to <6 years).
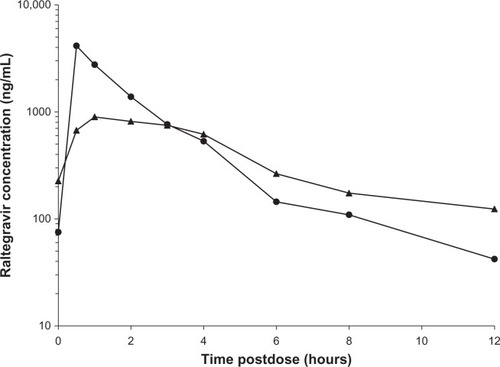
Table 1 Recommended doses and steady-state pharmacokinetic data for raltegravir in pediatric populationTable Footnotea
Table 2 Efficacy outcome of raltegravir in P1066 subjectsTable Footnotea
Table 3 Comparison between adult (BENCHMRK) study and pediatric efficacy studies of raltegravir