Figures & data
Figure 1 Adjuvant AI clinical trial designs. Various strategies by which AIs may be incorporated into adjuvant therapy have been investigated. Trials of AI monotherapy have typically randomized patients before commencing adjuvant therapy to either 5 years of AI or tamoxifen. The switching strategy entails a randomization to either tamoxifen or AI after 2.5–3 years of tamoxifen. Crucially, randomization at this time point (b) provides no information on events occurring before the switch. This differs from the sequencing trials in which randomization occurs at the initiation of adjuvant therapy (a). Extended adjuvant trials have randomized patients who have completed 5 years of adjuvant tamoxifen to an AI or not (c). As with the switch strategy, events occurring before randomization are not addressed.
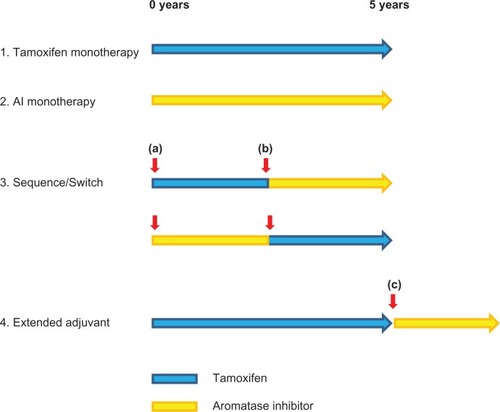
Table 1 Analyses of International exemestane study