Figures & data
Figure 1 Graphic representation of semaphorins and their receptors.
Notes: Semaphorins are divided into eight subclasses (classes 1–7 and class V). (A) All semaphorins are characterized by a sema domain and PSI domain. Among the vertebrate semaphorins, class 3 is secreted and classes 4, 5, and 6 are membrane bound, whereas class 7 semaphorins are GPI-anchor membrane-bound glycoproteins. Class 3 semaphorins have C-terminal basic-charged domain, which is required for binding to neuropilins. Several class 3, 4, and 7 semaphorins undergo controlled proteolytic cleavage by FPPCs or metalloproteases (ADAMTS1). Class 5 semaphorins contain thrombospondin repeats. (B) Neuropilins are single-pass transmembrane receptors that are characterized by the presence of two CUB domains, two FV/FVIII coagulation factor-like domains, and one MAM domain. (C) Integrins αV and β1 are characterized by the presence of a sema and PSI domains, respectively. (D) VEGFR possesses Ig-like and kinase domain. ErbB2 contains furin and receptor-L domains. (E) Plexins harbor one sema domain, two to three PSI domains and three IPT domains. The cytoplasmic domain of plexin is weekly similar to GAPs. In addition, the B-subfamily plexins contain PDZ domains. A cleavage site for FPPCs also exists in the extracellular domain of plexin-B.
Abbreviations: FPPCs, furin-like proprotein convertase; GAPs, GTP-ase activating proteins; GPI, glycophosphatidylinositol; IPT, Ig-like fold shared by plexin and transcription factors; PSI, plexin-semaphorin integrin; RTKs, receptor tyrosine kinases; VEGFR, vascular endothelial growth factor receptor.
Abbreviations: FPPCs, furin-like proprotein convertase; GAPs, GTP-ase activating proteins; GPI, glycophosphatidylinositol; IPT, Ig-like fold shared by plexin and transcription factors; PSI, plexin-semaphorin integrin; RTKs, receptor tyrosine kinases; VEGFR, vascular endothelial growth factor receptor.
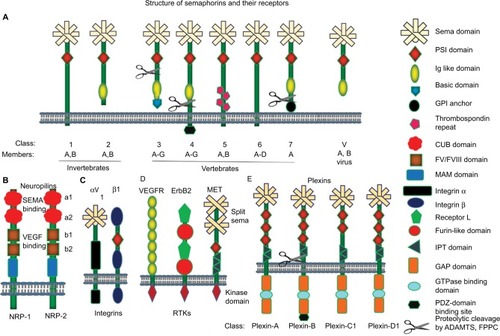
Figure 2 Semaphorin signaling in breast cancer.
Notes: Sema3A interacts with NRP1 receptor to induce PTEN/FOXO 3a-dependent MelCAM expression, which, in turn, inhibits tumor growth and angiogenesis. Sema3B binds to NRP1 and induces apoptosis by inhibiting PI3K/Akt signaling. Full-length Sema3C interacts with NRP2 on the lymphatic endothelial cells in tumor and suppresses lymphangiogenesis and metastasis by inhibiting VEGF-C–dependent ERK1/2 and Akt signaling. Full-length Sema3C undergoes proteolytic cleavage by FPPC to form p65-Sema3C, which promotes cancer cell survival. Sema4D binds to plexin-B1 and activates ErbB2, which, in turn, phosphorylates plexin-B1. Phosphorylated plexin-B1 induces migration by activating RhoA GTPase. Cleaved p61-Sema3E binds to plexin-D1 to promote metastasis through ErbB2-dependent MAPK signaling. Sema3E binds to plexin-D1 to inhibit apoptosis by disrupting the interaction between plexin-D1 and NR4A, which is known to induce caspase-9–mediated apoptosis. Sema7A interacts with integrin β1 on the cancer cells to promote invasion. Tumor-derived Sema7A binds with integrin β1 on the macrophages to promote angiogenesis by producing CXCL2, CXCL1, and MMP-9.
Abbreviations: ERK1/2, extracellular signal-regulated kinases1/2; FPPC, furin-like proprotein convertase; MMP, matrix metalloproteinase; PTEN, phosphatase and tensin homolog; VEGFR, vascular endothelial growth factor receptor.
Abbreviations: ERK1/2, extracellular signal-regulated kinases1/2; FPPC, furin-like proprotein convertase; MMP, matrix metalloproteinase; PTEN, phosphatase and tensin homolog; VEGFR, vascular endothelial growth factor receptor.
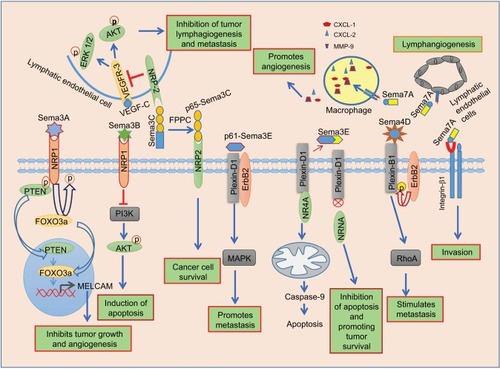
Table 1 Semaphorins, their receptors, and pathologic functions in breast cancer
