Figures & data
Table 1 Randomized trials evaluating chemotherapy and chemotherapy ± ovarian suppression
Table 2 Ovarian function suppression and outcome results
Figure 1 TEXT study description.
Abbreviations: EBC, early breast cancer; HR, hormone receptor; OFS, ovarian function suppression; TEXT, Tamoxifen and Exemestane Trial.
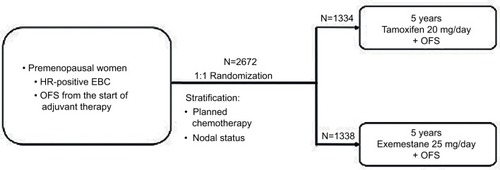
Figure 2 SOFT trial study description.
Abbreviations: EBC, early breast cancer; HR, hormone receptor; OFS, ovarian function suppression; SOFT, Suppression of Ovarian Function Trial.
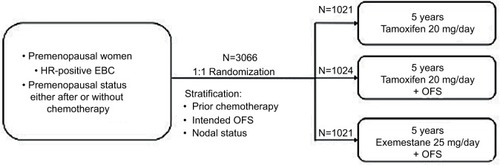
Table 3 Triptorelin and ovarian function preservation
