Figures & data
Figure 1 Fe3o4-pga-dha nanoparticles were obtained by loading dihydroartemisinin (DHA) onto polyglutamate-stabilized Fe3O4 magnetic nanoparticles (Fe3O4-PGA). Fe3o4-paspx-dox was obtained by combining DOX with Fe3O4-paspx magnetic nanoparticles (Fe3O4-paspx) loaded with polyaspartic acid. Two kinds of nano-drugs were injected into TME to release Fe2+ and DHA, Fe2+ and DHA induced ferroptosis through PL3K/AKT/mTOR/GPX4 pathway, and DOX promoted apoptosis of tumor cells, thereby jointly treating TNBC.
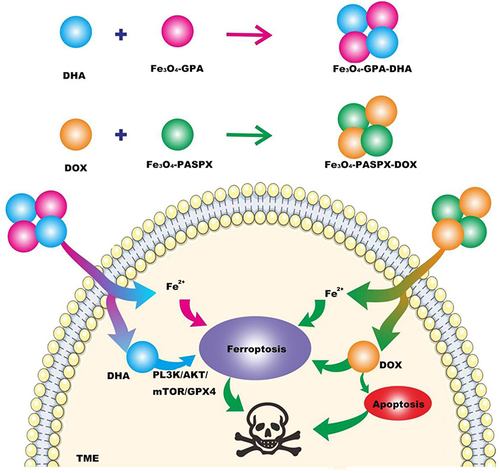
Figure 2 BSO is an inhibitor of gamma-glutamylcysteine synthetase (gamma-GCs), which can induce GSH depletion and thus inactivate GPX4 to promote ferroptosis. Gold nanoparticles (Au NPs) have the effect of sensitizing radiation. BSO/ZIF-8@Au@MPN@HA (BZAMH) nanomaterials were obtained by modifying BSO, Au NPs and ZIF-8 matrix with hyaluronic acid (HA) and injected into tumor microenvironment to decompose and release BSO to promote ferroptosis of tumor cells. Au NPs enhances the sensitivity of radiotherapy, thereby combining TNBC treatment.
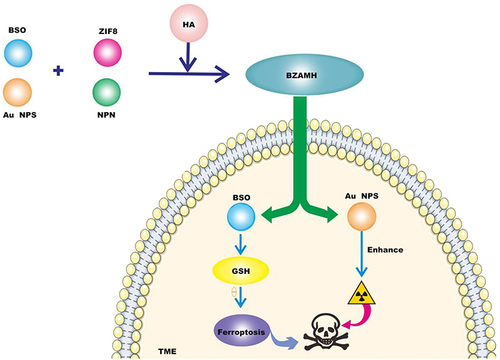
Figure 3 Sorafenib (SRF) and atovaquone (ATO) were loaded into bovine serum albumin (BSA) to prepare nanomaterials (ATO/SRF@BSA). ATO/SRF@BSA was injected into TME, and the ferroptosis inducer SRF was released to induce iron death of tumor cells. The release of targeted mitochondrial drug ATO down-regulates the defense mechanism of DHODH-Coenzyme Q (CoQH2), promotes the accumulation of lipid peroxides in mitochondria, triggers the explosion of lipid peroxides, and thus promotes ferroptosis of tumor cells.
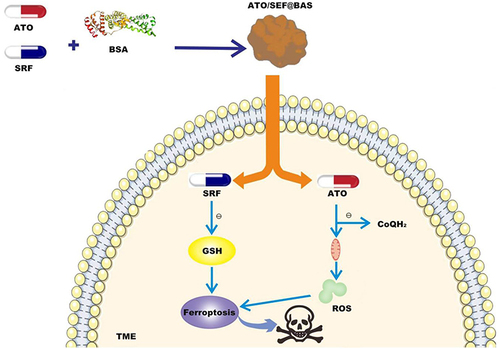
Figure 4 A kind of Ca & Mn dual-ion mixed nanomaterial (CMS) was constructed. In TME, the mixed valence of Mn in CMS leads to the consumption of GSH and the production of ROS to induce iron death, and Ca2+ induces iron death through Fenton reaction. CMS awakens innate immunity by alleviating intratumor hypoxia and activation of STING signaling pathway induced by Mn2+, promotes the polarization of macrophages (tam) from M2 phenotype to M1 phenotype, and effectively activates dendritic cells (dc) for antigen presentation and tumor cytotoxic T lymphocytes (ctl) infiltration into tumor tissues. Thus combined treatment of TNBC.
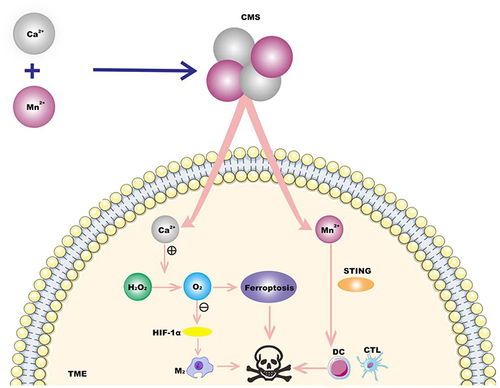
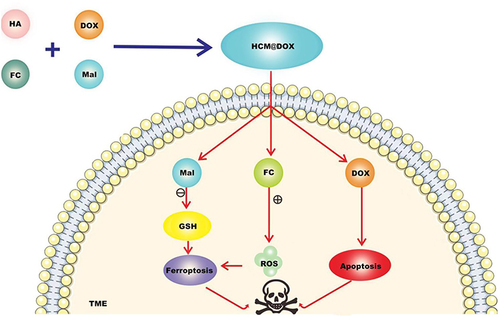
Figure 5 CT/CDT nanoparticles were prepared using functional materials and drugs such as hyaluronic acid (HA), maleimide caproic acid (Mal), ferrocene carboxylic acid (Fc) and DOX. They were released in TME, Mal consumed GSH and improved the efficiency of ferroptosis. Fc is a stable organometallic complex, and the oxidation state of iron atom in the center of Fc is Fe2+. Under high H2O2 conditions, ROS can induce ferroptosis of tumor cells, and DOX can induce ferroptosis and apoptosis, so as to combine TNBC treatment.
Data Sharing Statement
Data availability is not applicable to this article as no new data were created or analyzed in this study.
