Figures & data
Figure 1 Insulin molecule (hexamer).
Figure 2 Structures of human insulin (A) and the long-acting basal insulin analogs insulin glargine (B), insulin detemir (C), insulin lispro (D), insulin aspart (E), insulin glulisine (F), and insulin degludec (G).
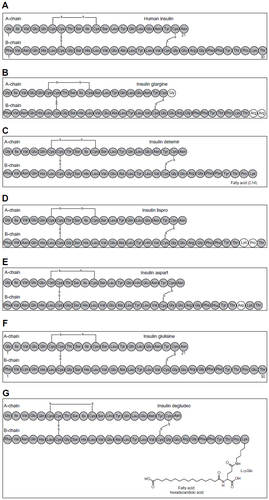
Table 1 Insulin glargine copy manufacturers
Figure 3 Sources of variation between manufacturers and batches of biosimilar products and reference originals.
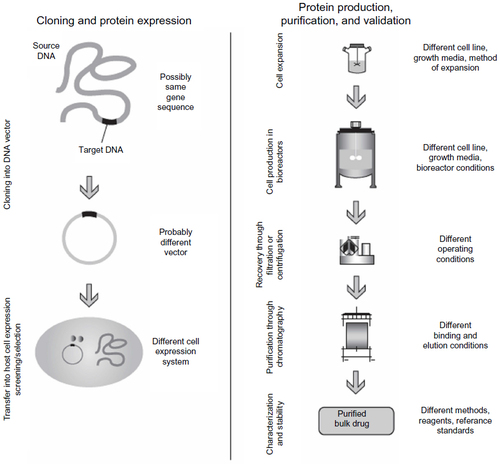
Table 2 Host systems for insulin manufacture
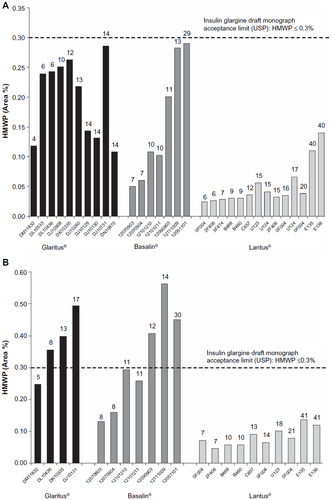
Figure 5 High-molecular-weight proteins (HMWP) in insulin glargine (original Lantus® and copy products) on direct testing (A) and after simulation of in-use period (28 days at +25°C) (B).
Abbreviation: USP, United States Pharmacopeia.