Figures & data
Table 1 Patient demographics at diagnosis and treatment before Ada
Figure 1 Kaplan–Meier analysis of probability of remaining on Ada therapy during follow-up.
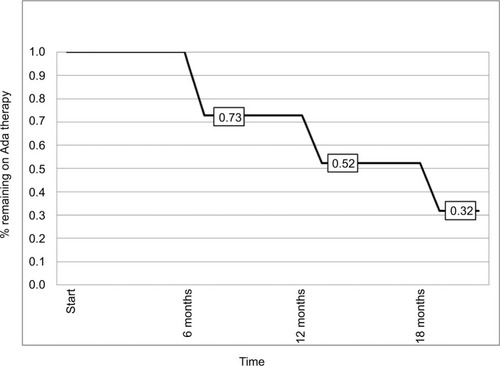
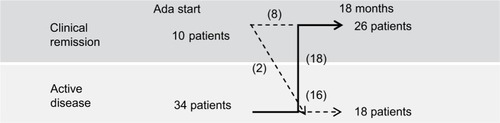
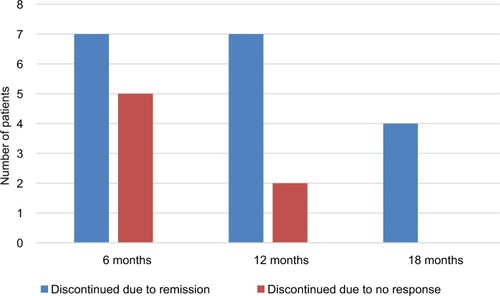
Figure 2 Rates of clinical remission, clinical response and failure of Ada in 44 children with Crohn’s disease on Ada therapy.
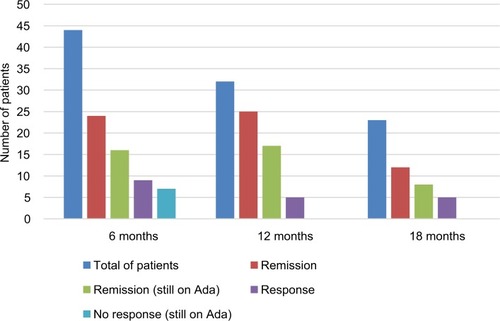
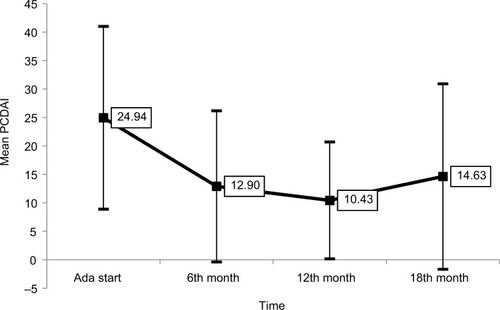
Figure 3 Mean PCDAI-value trend (with SD, P<0.01).