Figures & data
Figure 1 Signal 1 is antigenic, whereas signal 2 is costimulatory from the antigen-presenting cell.
Abbreviations: MHC, major histocompatibility complex; TCR, T-cell receptor.
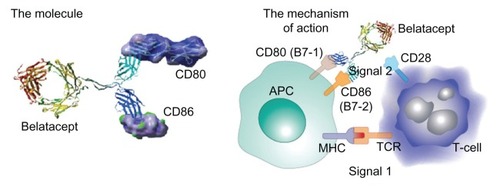
Figure 2 All patients received induction with basiliximab and maintenance therapy with mycophenolate mofetil and corticosteroids.
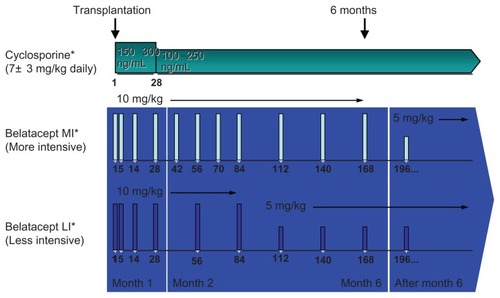
Figure 3 Measured glomerular filtration rate (GFR) by month 12 in patients with and without rejection in BENEFIT.
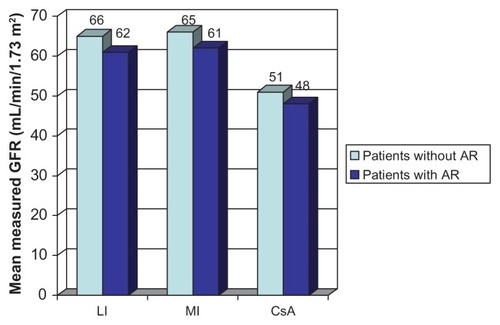
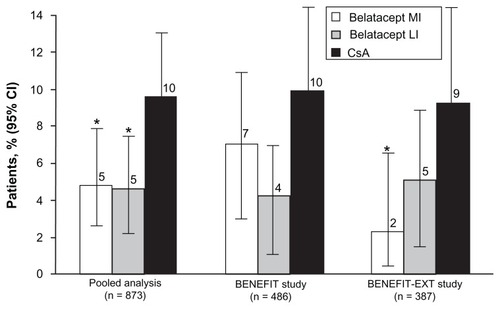
Figure 4 Percentage of patients at chronic kidney disease stages at 3 years in BENEFIT-EXT.
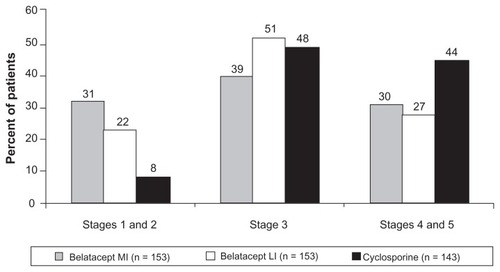
Table 1 Incidence of malignancies and PTLD in the pooled analysis from the Phase II and III belatacept trials