Figures & data
Figure 1 Schematic of the influenza HA epitope region and peptide sequences from the current study.
Abbreviations: F’, fusion peptide subdomain; E’, vestigial enzyme subdomain; R, receptor binding subdomain; HA, influenza hemagglutinin; N, N-terminal portion of the peptide; C, C-terminal portion of the peptide.
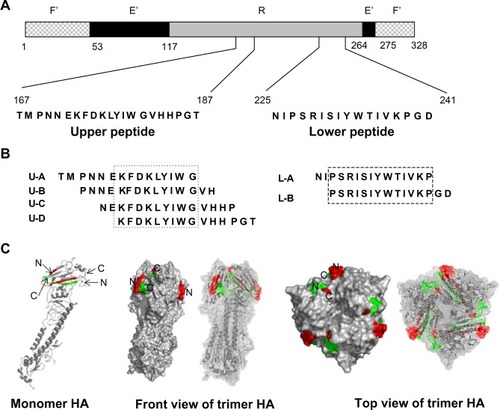
Figure 2 Binding activity of different rabbit antisera to epitope peptides, as determined by ELISA.
Abbreviations: aU, rabbit immunized with upper peptide alone; pre, serum harvested from each rabbit prior to immunization; n, number; aU+L, rabbit immunized with both the upper and lower peptides; aL, rabbit immunized with lower peptide alone; ELISA, enzyme-linked immunosorbent assay.
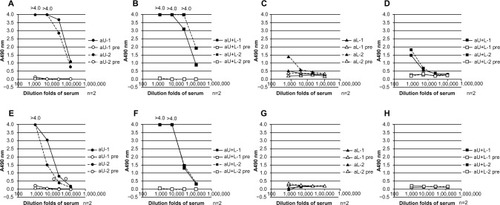
Figure 3 Binding ability of different rabbit antisera to HA, determined by ELISA and immunoblotting.
Abbreviations: n, number; aU, rabbit immunized with upper peptide alone; pre, serum harvested from each rabbit prior to immunization; aL, rabbit immunized with lower peptide alone; aU+L, rabbit immunized with both the upper and lower peptides; Bri, influenza hemagglutinin vaccine antigen derived from A/Brisbane/59/2007 (H1N1); Urg, influenza hemagglutinin vaccine antigen derived from A/Uruguay/716/2007 (H3N2); Flo, influenza hemagglutinin vaccine antigen derived from B/Florida/4/2006; HA, influenza hemagglutinin; ELISA, enzyme-linked immunosorbent assay; SDS-Page, sodium dodecyl sulfate polyacrylamide gel electrophoresis.
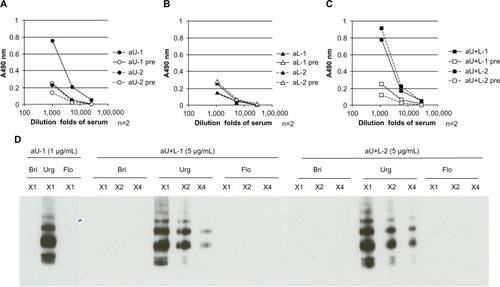
Figure 4 Binding abilities of antibodies affinity-purified with peptides to the HA vaccine, as determined by ELISA.
Abbreviations: aU, rabbit immunized with upper peptide alone; aU+L, rabbit immunized with both the upper and lower peptides; igg, immunoglobulin G; n, number; aL, rabbit immunized with lower peptide alone; HA, influenza hemagglutinin; ELISA, enzyme-linked immunosorbent assay.

Figure 5 Binding affinities to recombinant HA, as determined by ELISA.
Abbreviations: aU, rabbit immunized with upper peptide alone; aL, rabbit immunized with lower peptide alone; aU+L, rabbit immunized with both the upper and lower peptides; n, number; Upper-C, C-terminal region of the upper peptide; Upper-N, N-terminal region of the upper peptide; SD, standard deviation; Urg, A/Uruguay/716/2007; NY, A/New York/55/2004; HA, influenza hemagglutinin; ELISA, enzyme-linked immunosorbent assay.
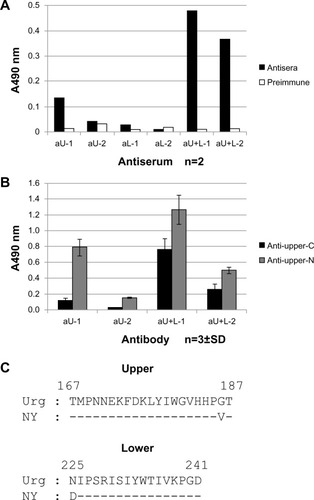
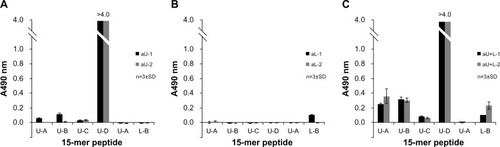
Figure 6 Binding activity of different rabbit antisera to 15-mer peptides within the upper and lower peptide regions of HA, as determined by ELISA.
Abbreviations: n, number; SD, standard deviation; HA, influenza hemagglutinin; ELISA, enzyme-linked immunosorbent assay; aU, rabbit immunized with upper peptide alone; aL, rabbit immunized with lower peptide alone; aU+L, rabbit immunized with both the upper and lower peptides.