Figures & data
Table 1 TAA peptides included as antigens in DPX-0907 therapeutic cancer vaccine and their sequences and species homologies
Figure 1 Intracellular IFN-γ in CD8+ T-cells (A) and IFN-γ producing cells (B and C) from draining LNs of HLA-A2 transgenic mice immunized with single- (A and B) or three-dose (C) vaccination, either in DPX-0907 or conventional emulsion vaccine. Draining LN cells from naïve and vaccinated mice were incubated with either unloaded or peptide-loaded DC and stained by intracellular immunofluorescence or in a DC-ELISPOT assay for detecting induction of IFN-γ.
Abbreviations: CD, cluster of differentiation; DC, dendritic cell; DPX, DepoVax™; ELISPOT, enzyme-linked immunospot; GM-CSF, granulocyte macrophage colony-stimulating factor; HLA, human leukocyte antigen; IFN, interferon; LN, lymph node; SFU, spot-forming unit.
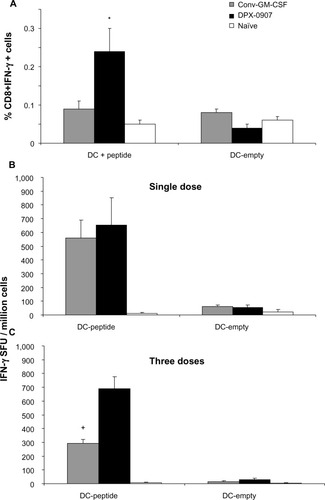
Figure 2 CD4+IL-10+ cells from spleen and CD4+IFN-γ+ cells from draining LNs of mice immunized with three doses of DPX-0907, oil emulsion vaccine or naïve mice. Splenocytes and LN cells from indicated groups of mice were isolated and activated for 48 hours in the presence of peptides and used for staining to detect suppressor Tr1 and effector CD4 T-cells.
Abbreviations: CD, cluster of differentiation; DPX, DepoVax™; IFN, interferon; IL, interleukin; LN, lymph node.
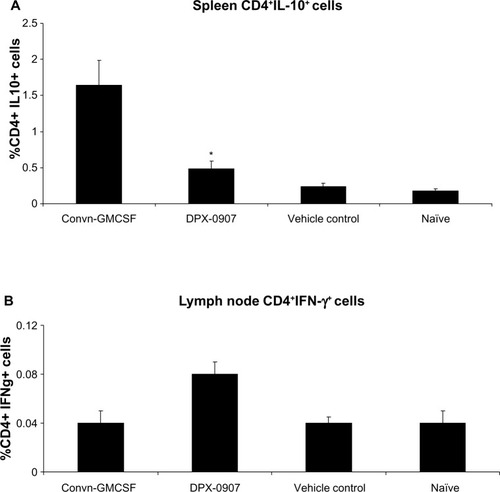
Figure 3 Outline of clinical protocol followed in DPX-0907 trial with details on the time-line for screening, treatment and follow-up of late-stage breast, ovarian, and prostate cancer patients. Blood collected at the indicated time points are used for immune monitoring, and the samples up to study day 73 are analyzed.
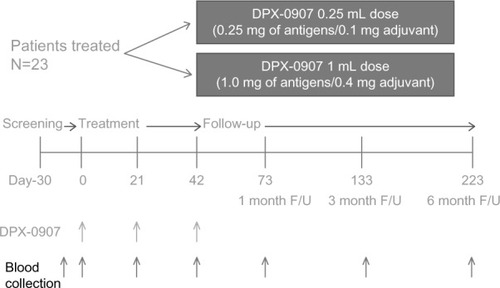
Table 2 Summary of the immune responses in DPX-0907-treated patients
Table 3 Responders among DPX-0907 vaccine recipients and their immune response to vaccine antigens as determined by MHC-multimer assay and multi-parametric flow cytometry
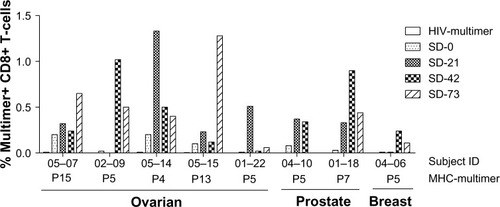
Figure 4 DPX-0907-induced increase in the frequency of antigen-specific CD8+ T-cells in vaccinated ovarian, prostate, and breast cancer patients. Patient PBMC were stimulated with indicated peptides for 10 days and were stained with corresponding MHC-multimer reagents to detect CD8+ T-cells with peptide-specific TCR repertoire. HIV-multimer served as a negative control and CMV-specific multimer was used on a known CMV-positive donor PBMC as internal positive control for the assay (data not shown).
Abbreviations: CD, cluster of differentiation; CMV, cytomegalovirus; DPX, DepoVax™; HIV, human immunodeficiency virus; MHC, major histocompatibility complex; PBMC, peripheral blood mononuclear cells; TCR, T-cell receptor; ID, identification number; SD, study day.