Figures & data
Table 1 Main three BoNTA products
Table 2 Comparison of manufacturing methods for BoNTA products
Figure 1 Comparison of clinical response of two hypothetical BoNTA products.
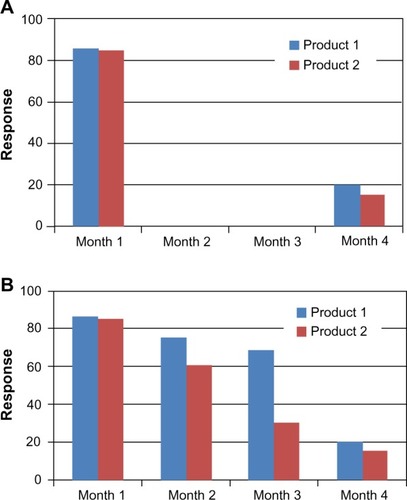
Table 3 Approved indicationsTable Footnotea for the main botulinum neurotoxin products available in the US and EUTable Footnoteb
Figure 2 Progressive differentiation of four hypothetical BoNTAs.
Abbreviation: BoNTA, botulinum toxin type A.