Figures & data
Table 1 Key characteristics and indications of Belotero® dermal fillers and hyaluronic acid fillers used as comparators in split-face, randomized controlled trials
Table 2 Belotero® dermal fillers: summary of the study design, methods, and main end points
Table 3 Performance assessment of dermal fillers: rating scales, and investigator’s and patient’s satisfaction
Table 4 Belotero® dermal fillers: summary of clinical findings
Figure 1 Two-dimensional surface profiles before (black lines) and 4 weeks after treatment (gray lines) with Belotero® Basic and Restylane®.
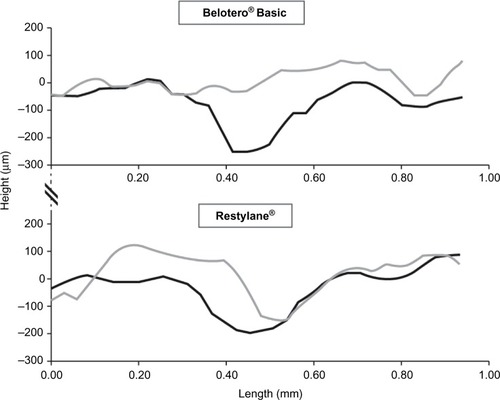
Figure 2 Change in nasolabial fold severity with Belotero® Basic/Balance treatment, re-treatment, and optional touch-ups.
Abbreviation: t-u, touch-up allowed.
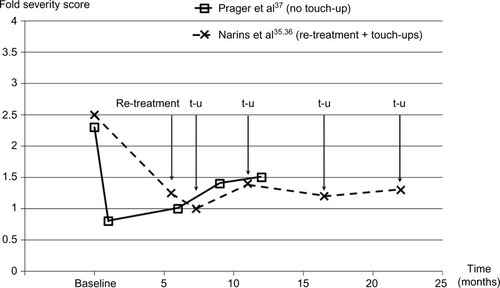
Figure 3 Esthetic effect of Belotero® Soft.
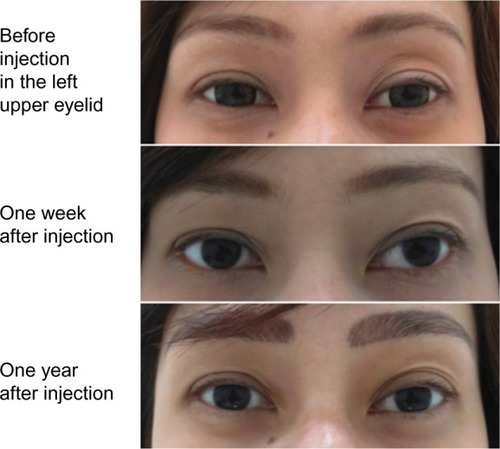
Figure 4 Long-term change in nasolabial fold severity with Belotero® Intense treatment based on the investigators’ rating on the Wrinkle Severity Rating Scale.
Abbreviation: WSRS, Wrinkle Severity Rating Scale.
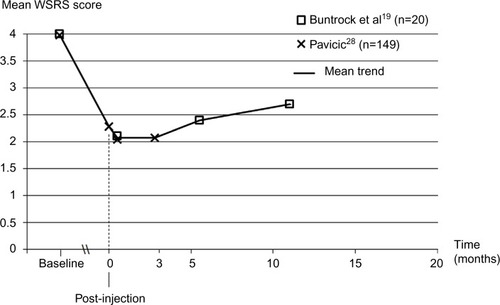
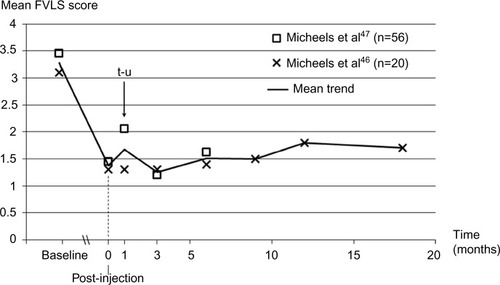
Figure 5 Long-term change in facial volume loss (cheeks) with Belotero® Volume treatment based on the investigators’ rating on the Facial Volume Loss Scale.
Abbreviations: FVLS, Facial Volume Loss Scale; t-u, touch-up allowed.