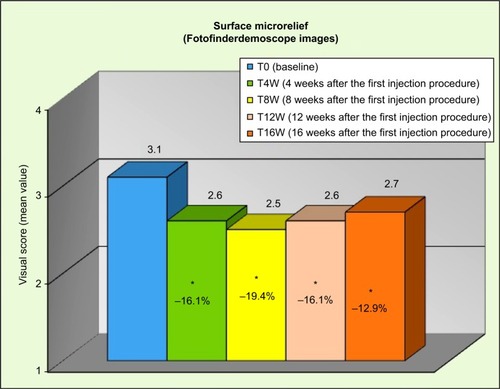
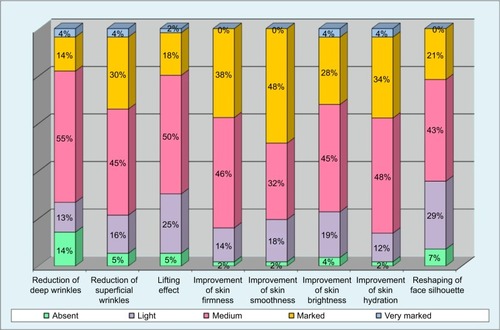
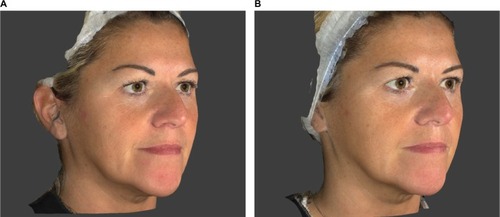
Figures & data
Table 1 Materials and instruments employed in the study
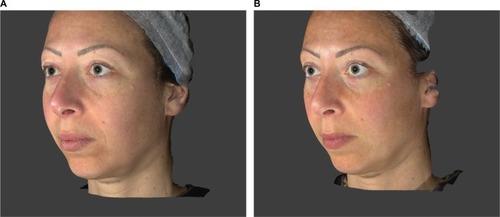
Figure 2 Reduction in the FVLS “cheek volume loss” throughout the study.
Abbreviation: FVLS, facial volume loss scale.
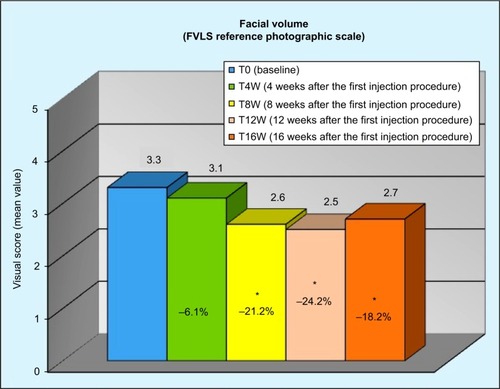
Figure 3 Reduction in the WSRS “wrinkle severity” throughout the study.
Abbreviation: WSRS, wrinkle severity rating scale.
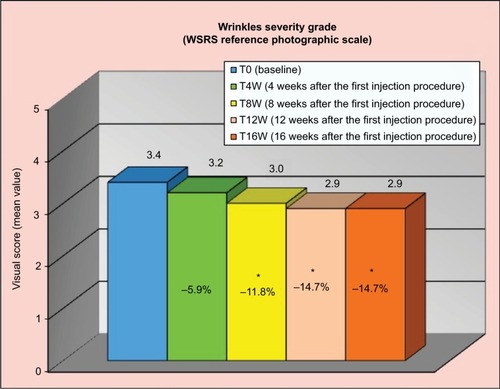
Figure 4 Variation from baseline in the skin electrical capacitance.
Abbreviation: AU, arbitrary unit.
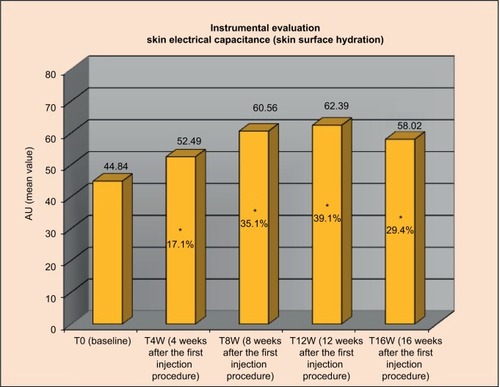
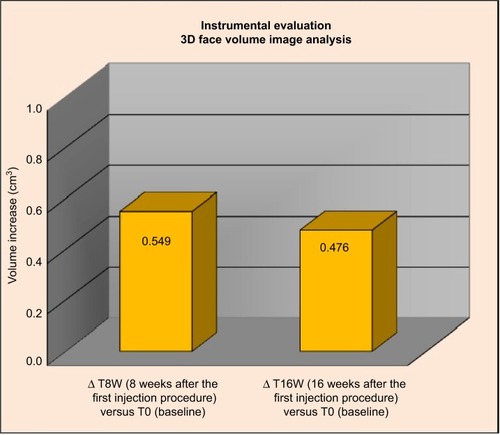
Figure 5 Three-dimensional face volume analysis: increase of volume (cm3) T0 versus T8W and T16W versus T0.