Figures & data
Table 1 Tolerance Evaluation Scale
Table 2 Demographics And Skin Characteristics At Inclusion In The Whole Study Population
Table 3 Acne History And Clinical Characteristics At Inclusion In The Whole Study Population
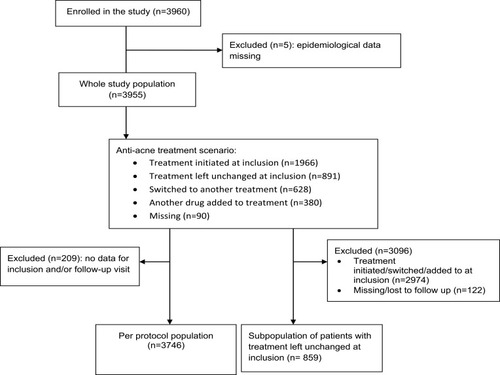
Figure 2 Severity of facial acne at inclusion and at follow-up after 2–3 months of use of the cosmetic product in combination with medical anti-acne therapy in the per protocol (PP) population (N=3746) and in the subpopulation of patients whose medical anti-acne treatment remained unchanged at inclusion (N=859). Severity was graded by the physician using the acne investigator’s global assessment (IGA) scale from 0 (clear) to 4 (severe). The percentages of patients with each score are shown.
Table 4 Acne Investigator’s Global Assessment And Objective Clinical Symptom Severity Scores In The Per Protocol Population And In The Subpopulation Of Patients Whose Medical Anti-Acne Treatment Remained Unchanged At Inclusion
Figure 3 Global effectiveness at follow-up after 2–3 months of use of the cosmetic product in combination with medical anti-acne therapy in the per protocol (PP) population (N=3746) and in the subpopulation of patients whose medical anti-acne treatment remained unchanged at inclusion (N=859). Global effectiveness was measured by the physician with a 6-point scale from 1 (total disappearance) to 6 (aggravation). The percentages of patients with each score are shown.
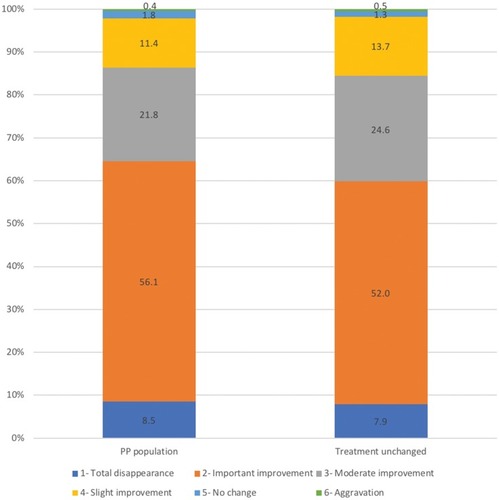
Table 5 Global Cardiff Acne Disability Index (CADI) Score And CADI Score Category At Inclusion And At The End Of Treatment In The Per Protocol Population And In The Subpopulation Of Patients Whose Medical Anti-Acne Treatment Remained Unchanged At Inclusion