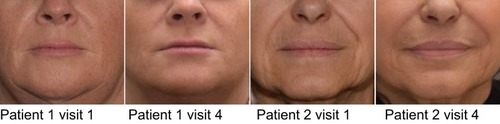
Figures & data
Figure 1 Croma-Pharma nasolabial folds severity rating scale.
Abbreviation: NLF, nasolabial fold.
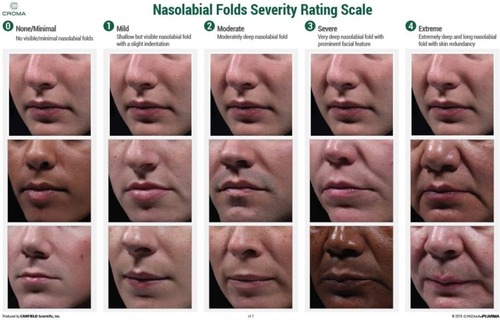
Figure 2 (A) Proportion of subjects with ≥1 grade improvement in NLF severity based on the NLF-SRS as assessed by the investigator and the independent reviewer of photographs (“reviewer”). (B) Proportion of subjects with ≥2 grades improvement in NLF severity based on the NLF-SRS as assessed by the investigator.
Abbreviations: N, number of subjects; NLF, nasolabial fold; NLF-SRS, nasolabial fold-severity rating scale.
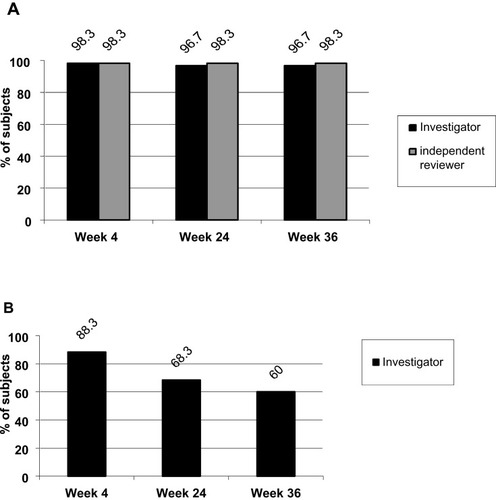
Table 1 (%) Of Subjects With ≥1 Grade Reduction Of NLF Severity By At Least 1 Grade On The NLF-SRS As Assessed By The Investigator
Figure 3 Proportion of subjects with improved aesthetics as assessed by the investigator using the GAIS.
Abbreviations: GAIS, Global Aesthetic Improvement Scale; N, number of subjects.
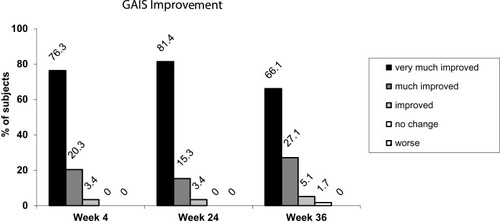
Figure 4 Subject satisfaction ratings.
Abbreviation: N, number of subjects.
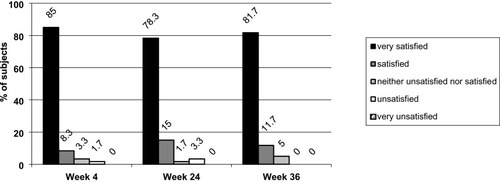
Figure 5 Pain score immediately after injection.
Abbreviation: N, number of subjects.
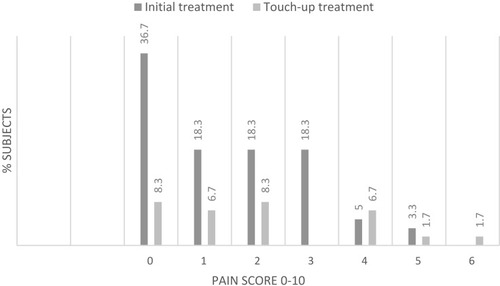
Table 2 Incidence Of ADEs Reported During The Investigation