Figures & data
Figure 2 Schematic model of proposed mechanisms of ingenol mebutate. (A) Ingenol mebutate penetrates skin in a gradient-dependent mannerCitation40 whereby it preferentially induces death in proliferating undifferentiated cellsCitation42 by increases in intracellular calcium, mitochondrial swelling, and loss of cell membrane integrity.Citation41 (B) Cell death and protein kinase C activation lead to an inflammatory activation of the treated field. Ingenol mebutate increases local production of tumor necrosis factor-alpha and interleukin-8, which recruit and activate neutrophilsCitation40,Citation47 and directly activate endothelial cell expression of the adhesion receptors E-selectin and intercellular adhesion molecule-1.Citation50
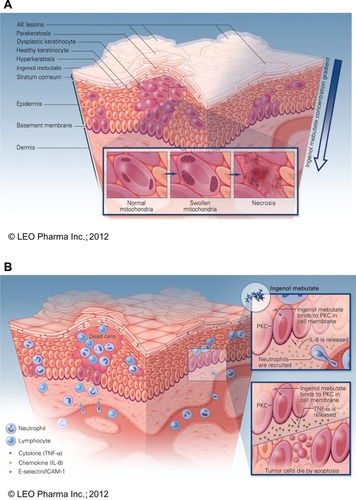
Figure 3 Treatment of skin in ultraviolet B–irradiated SKH1/hr mice with ingenol mebutate 0.05% gel reduced the number of skin lesions and mutant p53 patches that subsequently developed compared with placebo and no treatment. After 30 doses of ultraviolet B radiation, mice were untreated (control) or treated with placebo gel or ingenol mebutate 0.05% gel on days 0 and 2. (A) Representative mice from ingenol mebutate, placebo, and control groups taken at 21 weeks after treatment initiation. (B) The number of mutant p53 patches analyzed by immunohistochemistry in the skin of ultraviolet B–irradiated mice. Each symbol represents an individual mouse, and bars indicate the mean ± standard error of the mean.
Copyright © 2012. Nature Publishing Group. Reprinted with permission from Cozzi SJ, Ogbourne SM, James C, et al. Ingenol mebutate field-directed treatment of UVB-damaged skin reduces lesion formation and removes mutant p53 patches. J Invest Dermatol. 2012;132(4):1263–1271.Citation53
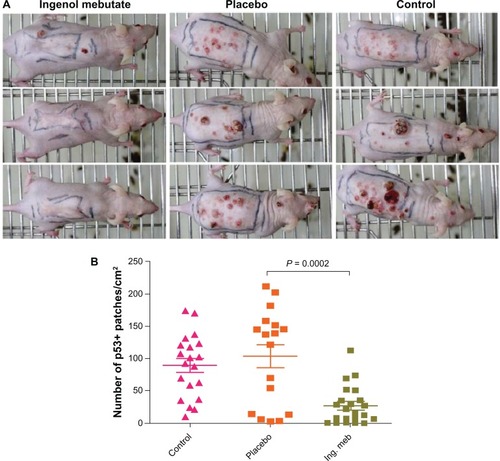
Table 1 Proportion of patients with complete or partial clearance and median reduction in number of lesions at day 57 (pooled analyses)
Figure 4 Time course of mean (± standard error of the mean) composite score for local skin reactions from pooled analyses at each study visit.
Copyright © 2012. Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society from Lebwohl M, Swanson N, Anderson LL, Melgaard A, Xu Z, Berman B. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366(11):1010–1019.Citation57
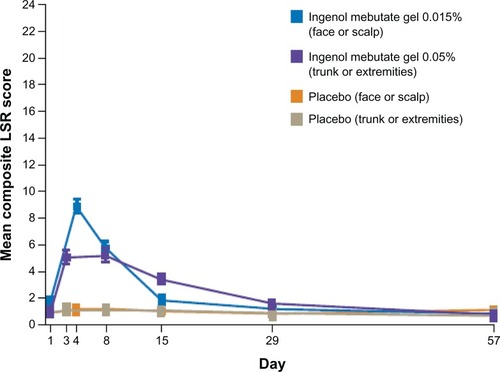
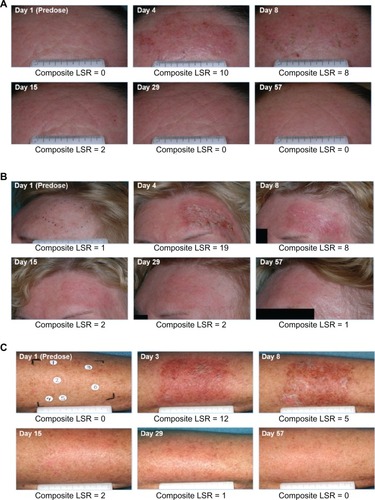
Figure 5 Clinical photographs of three patients. Photographs were taken at screening, before treatment on day 1, and on days 3 or 4 (one day after last treatment), 8, 15, 29, and 57. The composite local skin reaction score was calculated at each visit. (A) Forehead lesions. Intermediate peak composite local skin reaction score (10) on day 4 returned to baseline by day 29. (B) Forehead lesions. High peak composite local skin reaction score (19) on day 4 returned to baseline by day 57. (C) Arm lesions. Intermediate peak composite local skin reaction score (12) on day 3 returned to baseline by day 57.
Data on file. Clinical study reports PEP005-016 and PEP005-028. Parsippany, NJ: LEO Pharma Inc.; 2010.