Figures & data
Figure 1 Skin sensitivity at baseline, Day 15 and Day 30. M89PF significantly (p<0.0001) reduced skin sensitivity as early as Day 15.
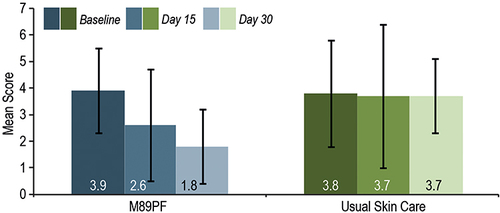
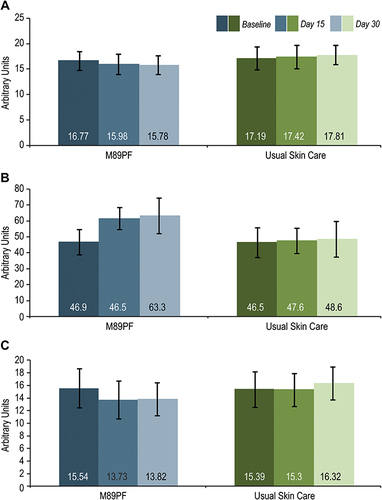
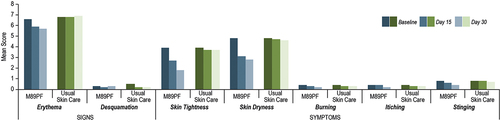
Figure 2 Instrumental assessments at baseline, Day 15 and Day 30. M89PF significantly (p<0.0001) improved erythema, skin hydration and transepidermal water loss as early as Day 15 compared to the standard skin care. (A) Erythema. (B) Skin hydration. (C) Transepidermal water loss.