Figures & data
Figure 1 Comparison of syringe-harvested isolated adipose-derived mesenchymal stromal cell (AD-MSC) counts (open circles) versus use of low-pressure machine harvest (wall suction or detuned lipoaspiration machine [dark circles]).
Abbreviation: OD, outside diameter.
![Figure 1 Comparison of syringe-harvested isolated adipose-derived mesenchymal stromal cell (AD-MSC) counts (open circles) versus use of low-pressure machine harvest (wall suction or detuned lipoaspiration machine [dark circles]).](/cms/asset/fb64aa63-8dce-4f73-b5a8-44cb9679b99a/dcci_a_40575_f0001_b.jpg)
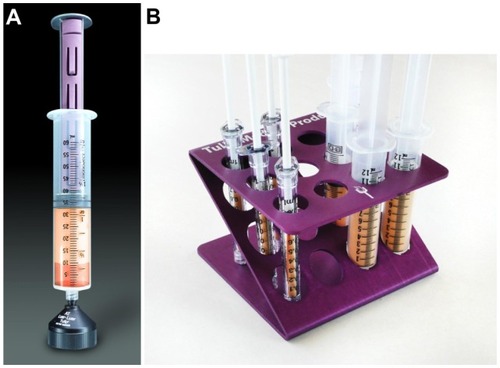
Figure 2 Complete closed-syringe microcannula system (Tulip® GEMS™, Tulip Medical Systems, San Diego, CA, USA).
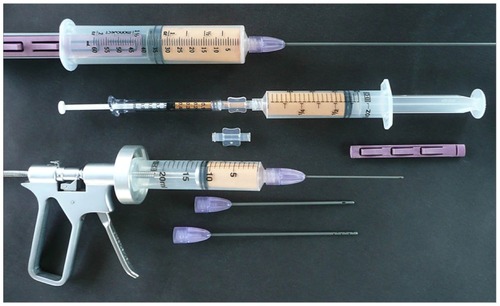
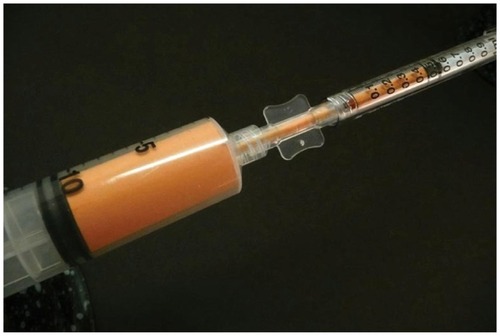
Figure 3 Tulip® super luer-lock connection (Tulip Medical Systems, San Diego, CA, USA).

Figure 4 Tulip® (Tulip Medical Systems, San Diego, CA, USA) cell-friendly (autoclavable) microcannulas.

Figure 5 Disposable microcannula cannulas for closed-syringe lipoaspiration of small-volume autologous adipose grafting (Tulip® GEMS™, Tulip Medical Systems, San Diego, CA, USA).
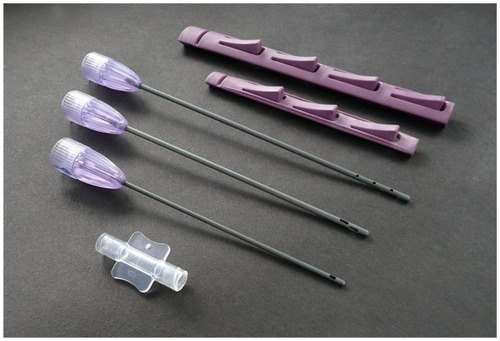
Figure 6 Close-up of microcannula openings.
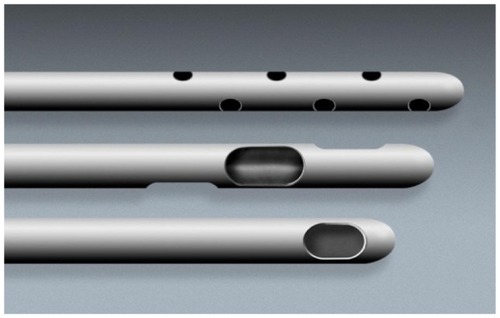
Figure 7 Closed-syringe lock options.
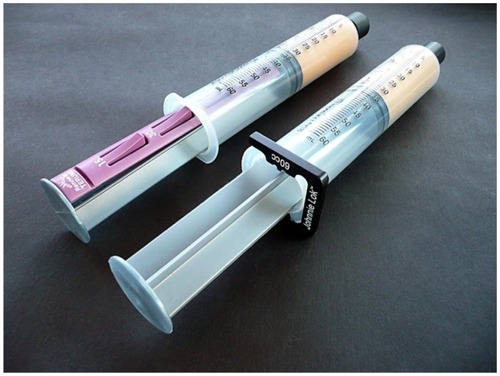
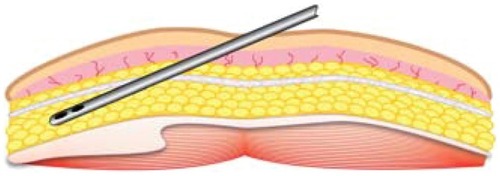
Figure 10 Example marking of lower abdomen site for harvesting of subdermal adipose tissue.
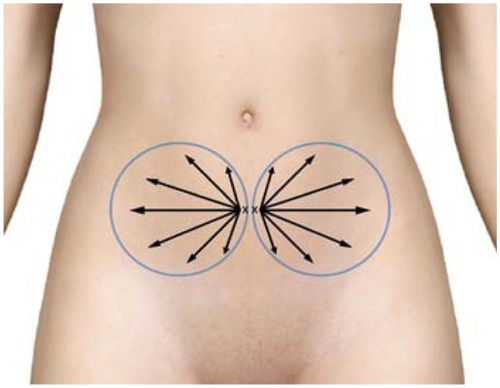
Figure 13 The SmartPRep®2 APC+™centrifuge, which forms part of the AdiPRep™ Adipose Transfer System (Harvest-Terumo, Plymouth, MA, USA).

Figure 14 Close-up of a disposable processing syringe containing extracted tissue that has separated.

Figure 15 Anaerobic transfer from disposable processing syringe (above) to adipose fat graft syringe (below).
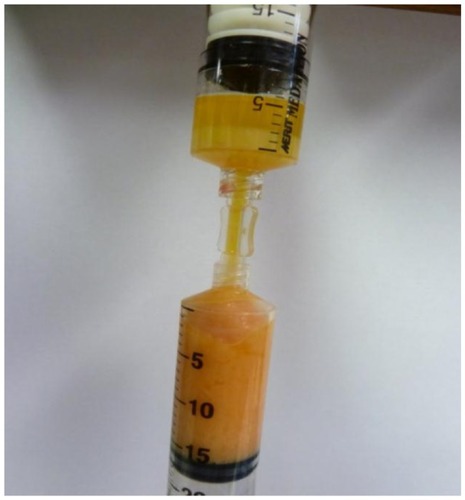
Figure 16 Tulip® GEMS™ (Tulip Medical Systems, San Diego, CA, USA) single-port injection cannulas (top) and close-up of the tip of one of these (bottom).

Figure 17 (A) Close-up of closed-cell compression foam (TenderFoam™; T&N Industries, San Diego, CA, USA). (B) Foam in place prior to firm compression.
Figure 18 Clinical examples. Lips (A) pre- and (B) 1-year post-augmentation (autologous fat grafting [AFG] plus high-density platelet-rich plasma [HD PRP] [upper lip 3 cc; lower lip 2 cc]). (C) Pre- and (D) postoperative (20 months) AFG plus HD PRP grafting to lips, cheeks and nasolabial folds (lips: upper 2.5 cc; lower 2 cc; cheeks: 5 cc bilateral, malar and sub-malar placement; nasolabial folds: 3 cc bilateral). Cheeks (E and F) pre- and (G and H) 2 years post-AFG plus HD PRP (bilateral cheeks; total volume grafted, 5 cc bilateral malar and sub-malar) (Photos with permission). (I) Pre- and (J) postoperative (4 years) large-volume augmentation of both breasts (closed-syringe technique, cell-friendly cannulas) using AFG plus HD PRP concentrate; right, 300 cc; left, 325 cc).
![Figure 18 Clinical examples. Lips (A) pre- and (B) 1-year post-augmentation (autologous fat grafting [AFG] plus high-density platelet-rich plasma [HD PRP] [upper lip 3 cc; lower lip 2 cc]). (C) Pre- and (D) postoperative (20 months) AFG plus HD PRP grafting to lips, cheeks and nasolabial folds (lips: upper 2.5 cc; lower 2 cc; cheeks: 5 cc bilateral, malar and sub-malar placement; nasolabial folds: 3 cc bilateral). Cheeks (E and F) pre- and (G and H) 2 years post-AFG plus HD PRP (bilateral cheeks; total volume grafted, 5 cc bilateral malar and sub-malar) (Photos with permission). (I) Pre- and (J) postoperative (4 years) large-volume augmentation of both breasts (closed-syringe technique, cell-friendly cannulas) using AFG plus HD PRP concentrate; right, 300 cc; left, 325 cc).](/cms/asset/bbd7ced5-d61b-41aa-bd1e-bfb10064d16e/dcci_a_40575_f0018_c.jpg)