Figures & data
Table 1 Baseline Demographics and Clinical Characteristics of the Patients
Table 2 Tuberculosis and Hepatitis B Screening Outcomes at Week 28 of Patients Combined with LTBI or Inactive HBV Infection at Baseline
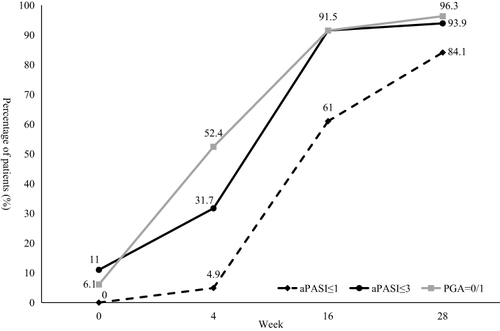
Figure 1 PASI 75 and PASI 90 response rate (A) and percentage of PASI improvement (B) during the 28-week treatment period.
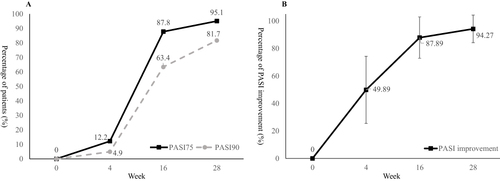
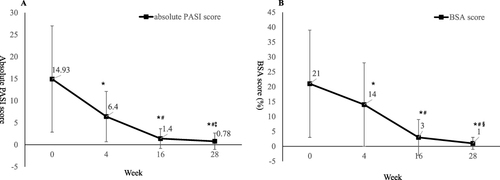
Figure 2 Change of absolute PASI score (A) and BSA score (B) during the 28-week treatment period. *P<0.001 compared with baseline; #P<0.001 compared with week 4; ‡P<0.001 compared with week 16; §P=0.001 compared with week 16.
Data Sharing Statement
The data of this study can be available from Dr Wen-Sheng Lu ([email protected]) for reasonable request.