Figures & data
Table 1 Overview of Phase II and III clinical studies with BT in patients with moderate to severe erythema of rosacea
Table 2 CEA and PSA
Figure 1 Percentage of subjects treated with brimonidine tartrate with improvements on CEA and PSA.
Abbreviations: n, number; CEA, clinician’s erythema assessment; PSA, patient’s self-assessment.
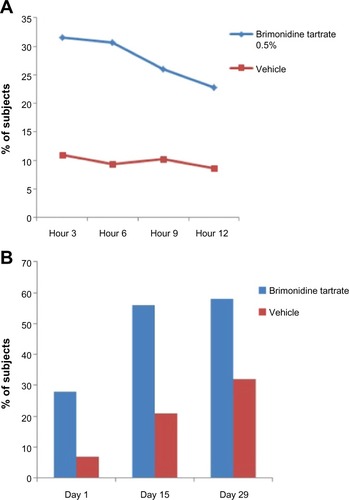
Figure 2 Standardized photos of a representative subject before and at 30 minutes, 3 hours, 6 hours, 9 hours, and 12 hours after the application of brimonidine tartrate gel on day 1.
Abbreviations: CEA, clinician’s erythema assessment; PSA, patient’s self-assessment.
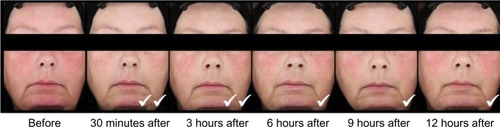
Figure 3 Standardized photos of a representative subject before and at 30 minutes, 3 hours, 6 hours, 9 hours, and 12 hours after the application of brimonidine tartrate gel on day 1.
Abbreviations: CEA, clinician’s erythema assessment; PSA, patient’s self-assessment.
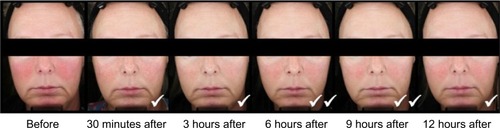
Table 3 Most commonly reported adverse events considered to be related to the study drug (≥2% patients)
