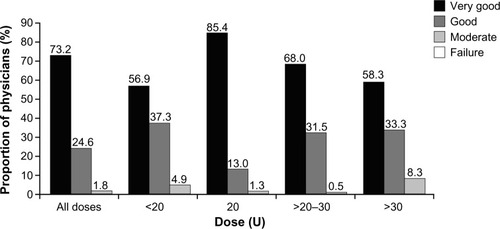
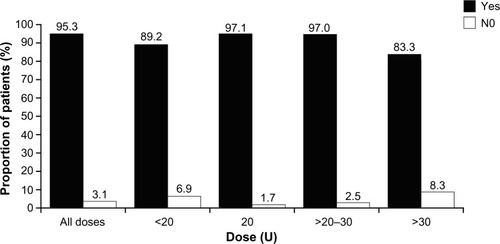
Figures & data
Table 1 Patient (n=638) and physician (n=66) characteristics
Table 2 Treated indicationsTable Footnotea
Figure 1 Time intervals between incobotulinumtoxinA injections to treat glabellar frown lines.
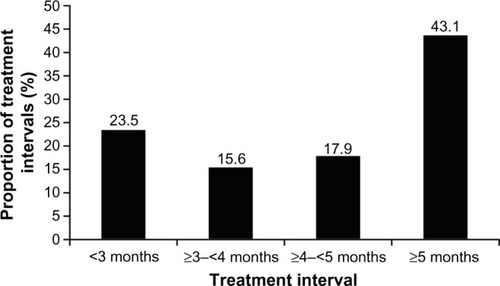
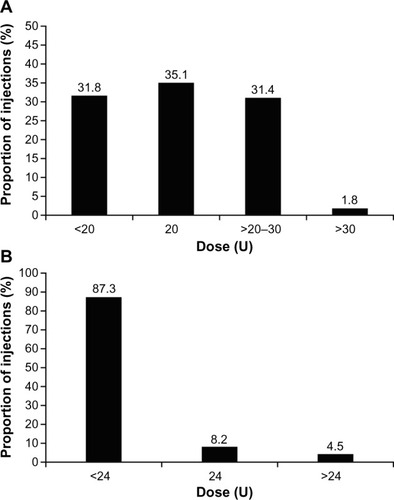
Figure 2 Dose of incobotulinumtoxinA injected to treat (A) glabellar frown lines and (B) crow’s feet.