Figures & data
Core evidence clinical impact summary
Figure 1 The scheme of the trastuzumab and pertuzumab action. Trastuzumab binds to the extracellular domain (ECD) IV of the HER2 receptor, preventing not only the spontaneous formation of homodimers (HER2–HER2) but also ligand-independent heterodimers (HER2–HER3, HER2–HER1, and HER2–HER4). Pertuzumab binds to the dimerization domain of the HER2 receptor (ECD II), preventing ligand-dependent HER2 heterodimerization. Adapted by permission from the American Association for Cancer Research: Metzger-Filho O, Winer EP, Krop I. Pertuzumab: Optimizing HER2 blockade. Clin Cancer Res. 2013;19(20):5552–5556. doi:10.1158/1078-0432.CCR-13-0518.Citation30
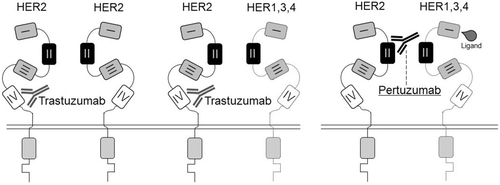
Table 1 Summary of the CLEOPATRA, APHINITY, NeoSphere, and TRYPHAENA clinical trials
Table 2 Primary adverse effects reported from the CLEOPATRA, APHINITY, NeoSphere, and TRYPHAENA trial
Table S1 Ongoing clinical trials of pertuzumab for breast cancer patients
