Figures & data
Core Evidence clinical impact summary for Regorafenib/liver cancer therapy
Figure 1 Barcelona Clinic Liver Cancer staging system and treatment strategy.
Abbreviations: RF, radiofrequency ablation; PEI, percutaneous ethanol injection; TACE, transcatheter arterial chemoembolization.
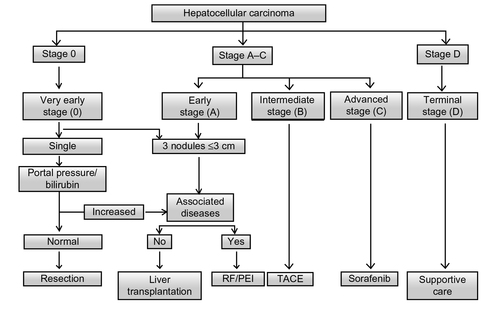
Figure 2 Pathways involved in the development of hepatocellular carcinoma.
Abbreviations: IGF, insulin-like growth factor; TGF, transforming growth factor; VEGF, vascular endothelial growth factor; EGF, epidermal growth factor; FGF, fibroblast growth factor; PTEN, phosphatase and tensin homologue.
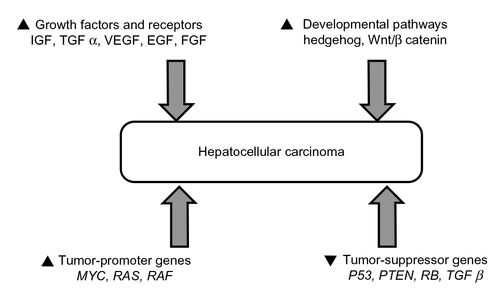
Figure 3 Structure of Regorafenib. 4-(4-[{(4-Chloro-3-[trifluoromethyl]phenyl)carbamoyl}amino]-3-fluorophenoxy)-N-methylpyridine-2-carboxamide.
![Figure 3 Structure of Regorafenib. 4-(4-[{(4-Chloro-3-[trifluoromethyl]phenyl)carbamoyl}amino]-3-fluorophenoxy)-N-methylpyridine-2-carboxamide.](/cms/asset/1a217696-0120-4fc5-96f8-8cf06c994356/dcev_a_48626_f0003_b.jpg)
Table 1 Biochemical activity of regorafenib and sorafenib: target inhibition
Table 2 Adverse-effect profile of Regorafenib
