Figures & data
Figure 1 Patient disposition.
Abbreviation: ITT, intent-to-treat.
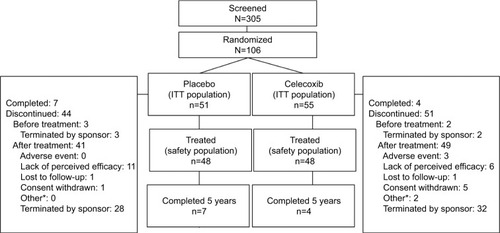
Table 1 Baseline patient characteristics (ITT population)
Figure 2 Time to disease progression (ITT population).
Abbreviation: ITT, intent-to-treat.
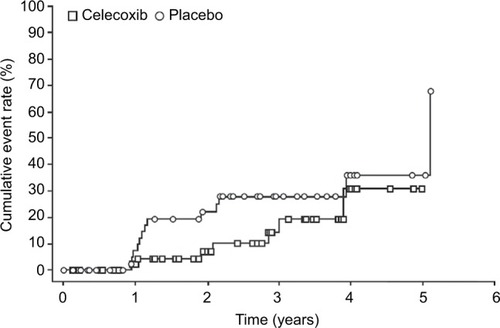
Table 2 Total number of colorectal polyps (>2 mm in size) detected at annual colonoscopies findings (ITT population)
Table 3 Patients with AEs (safety population)Table Footnote*
Table 4 AEs occurring in ≥10% of patients per group (safety population)
