Figures & data
Table 1 Subject demographics and other baseline characteristics
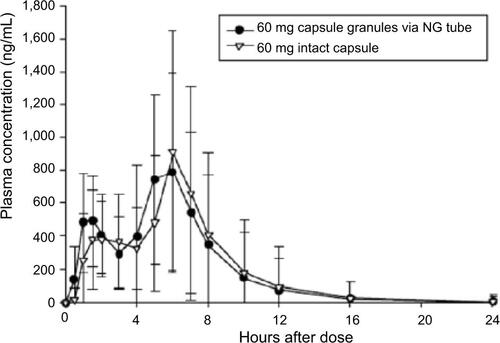
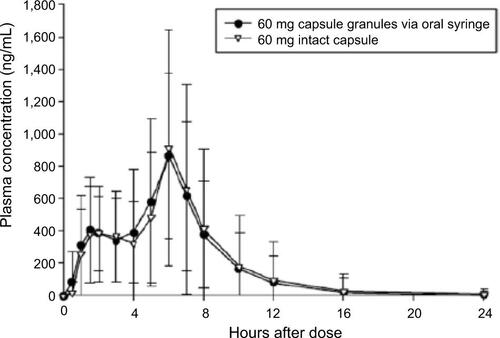
Figure 1 Mean dexlansoprazole plasma concentration–time profiles (linear format) following a single dose of 60 mg dexlansoprazole capsule as the intact capsule or as granules mixed with water and administered via NG tube or orally via syringe.
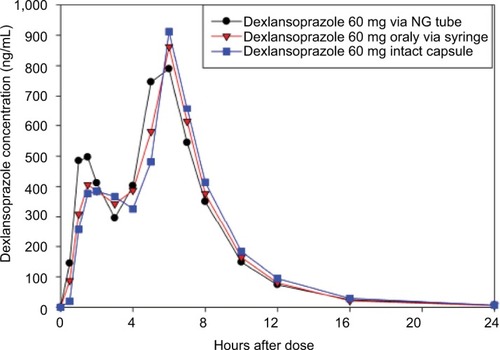
Table 2 Dexlansoprazole pharmacokinetic parameters following administration of dexlansoprazole 60 mg as an aqueous mixture of capsule granules via an NG tube, orally by syringe, or as the intact capsule to healthy participants
Table 3 Statistical comparisons of pharmacokinetic parameter estimates for dexlansoprazole following administration of dexlansoprazole 60 mg as an aqueous mixture of capsule granules via NG tube or orally via syringe, or as the intact capsule to healthy participants
Table 4 TEAEs, including SAEs