Figures & data
Table 1 Therapies for the management of patients with IBS-D
Table 2 Summary of meta-analyses of randomized, controlled studies of antibiotics and probiotics in patients with IBS
Figure 1 Repeat treatment trial study design and efficacy outcomes.Citation53
Abbreviations: EOS, end of study; tid, three times daily.
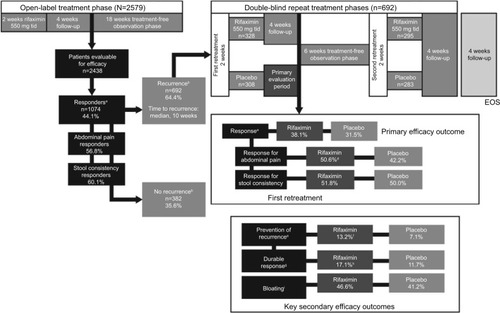
Table 3 Summary of safety of rifaximin 550 mg with nonconstipation IBSTable Footnotea
Table 4 Benefit to harm evaluation of treatment for patients with IBS-DTable Footnotea,Table Footnoteb
