Figures & data
Figure 1 Role of the intestinal barrier components in a healthy gut and a leaky gut.
Abbreviations: IAP, intestinal alkaline phosphatase; LPS, lipopolysaccharide; M cell, microfold cell; SCFA, short-chain fatty acid.
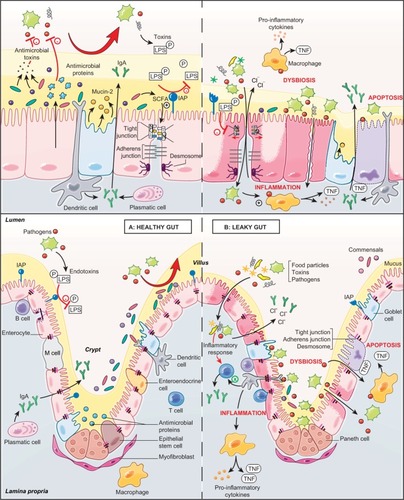
Table 1 Deleterious actions of pathogens in the gut and beneficial effects of S. boulardii CNCM I-745
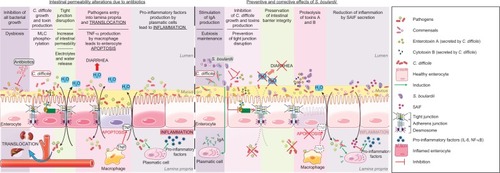
Figure 2 Proposed model for the effects of S. boulardii on intestinal permeability due to antibiotics.
Abbreviations: C. difficile, Clostridium difficile; MLC, myosin light chain; NF-κB, nuclear factor-κB; S. boulardii, Saccharomyces boulardii; SAIF, S. boulardii anti-inflammatory factor.