Figures & data
Figure 1 Mean intragastric pH from 0 to 24 hours postdose after single oral doses of dexlansoprazole modified-release 60 mg (n = 43) and esomeprazole 40 mg (n = 44) delayed-release capsules.
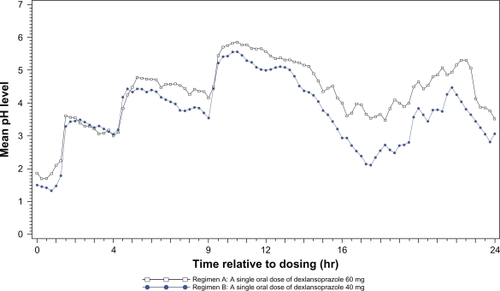
Figure 2 Mean percentage of time with intragastric pH > 4.0 at 0–24 hours, 0–12 hours, and >12–24 hours after single oral doses of dexlansoprazole modified-release 60 mg and esomeprazole 40 mg delayed-release capsules (n = 43). Only subjects who had valid pharmacodynamic parameters estimated for both periods were included in the pharmacodynamic analyses for that parameter.
Notes: *P ≤ 0.05; **P < 0.01; ***P < 0.001.
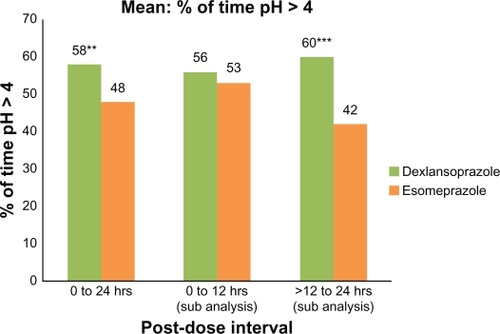
Figure 3 Mean intragastric pH at 0–24 hours, 0–12 hours, and >12–24 hours after single oral doses of dexlansoprazole modified-release 60 mg and esomeprazole 40 mg delayed-release capsules (n = 43). Only subjects who had valid pharmacodynamic parameters estimated for both periods were included in the pharmacodynamic analyses for that parameter.
Notes: *P ≤ 0.05; **P < 0.01; ***P < 0.001.
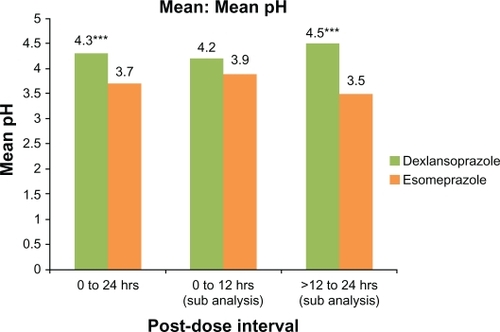
Figure 4 Mean plasma concentration-time curves of dexlansoprazole and esomeprazole after single oral doses of dexlansoprazole modified-release 60 mg (n = 43) and esomeprazole 40 mg (n = 44) delayed-release capsules in healthy subjects, linear scale.
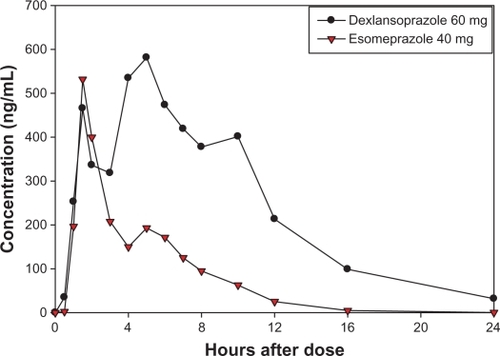
Table 1 Summary of plasma pharmacokinetic parameters for dexlansoprazole and esomeprazole after single oral doses of dexlansoprazole MR 60 mg and esomeprazole 40 mg