Figures & data
Table 1 Summary of randomized controlled trials of everolimus in liver transplantation
Figure 1 Incidence of biopsy-proven acute rejection at month 12 in comparative trials of everolimus.
Abbreviations: EVR, everolimus; CNI, calcineurin inhibitor; BPAR, biopsy-proven acute rejections; NR, not reported; NS, not significant.
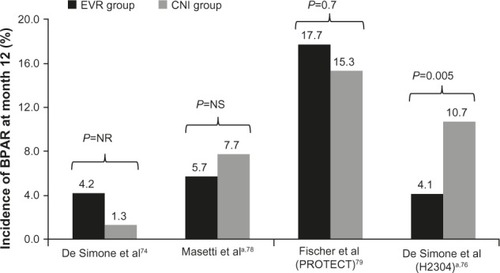
Figure 2 Mean estimated glomerular filtration rate with everolimus + reduced tacrolimus versus tacrolimus control in the H2304 study.
Abbreviations: EVR, everolimus; TAC, tacrolimus; eGFR, estimated glomerular filtration rate.
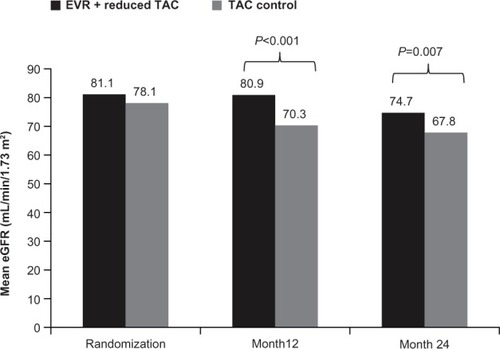
Figure 3 Key adverse events with everolimus + reduced tacrolimus versus tacrolimus control in the H2304 study.
Abbreviations: AE, adverse event; CI, confidence interval; CMV, cytomegalovirus; CV, cardiovascular; EVR, everolimus; GI, gastrointestinal; HCC, hepatocellular carcinoma; ILD, interstitial lung disease; NODM, new-onset diabetes mellitus; TAC, tacrolimus.
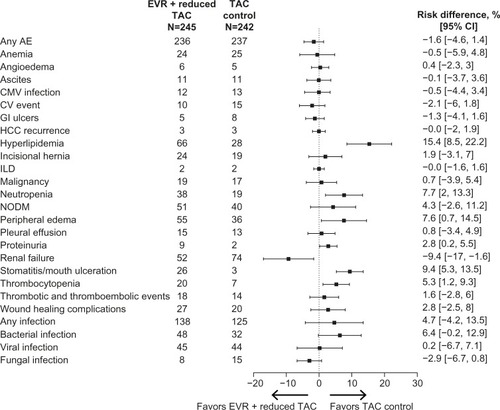
Table 2 Summary of key ongoing trials of everolimus in liver transplantation
