Figures & data
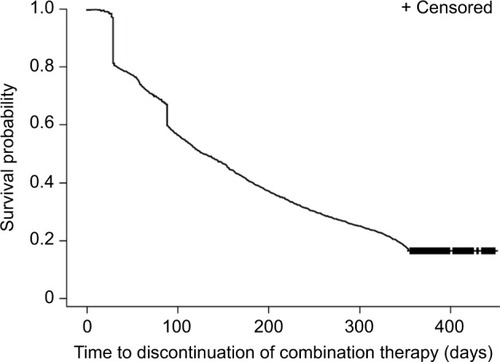
Figure 1 (A) Participant flow chart. (B) Schematic of study design.
Abbreviations: T2D, type 2 diabetes; GLP-1, glucagon-like peptide-1.
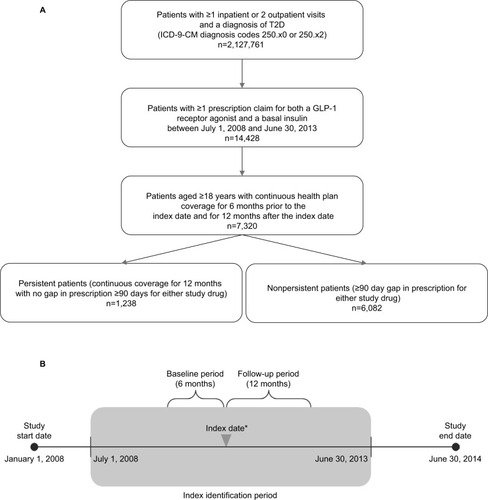
Table 1 Baseline demographic and clinical characteristics
Table 2 Health care utilization in the 12-month follow-up period
Figure 3 All-cause (A) and diabetes-related (B) health care costs over the 12 months of follow-up.
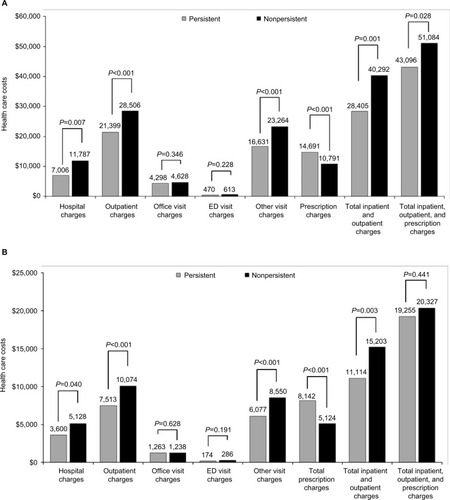
Table 3 Predictors of A1C change