Figures & data
Table 3 Annual transition probabilities
Table 1 Baseline characteristics of patient population
Table 2 SVR values applied in the model
Table 4 Utilities and costs used in the model
Table 5 Discounted base case results for treatment-experienced patients (F3/F4)
Figure 1 Univariate sensitivity analysis: testing versus no testing.
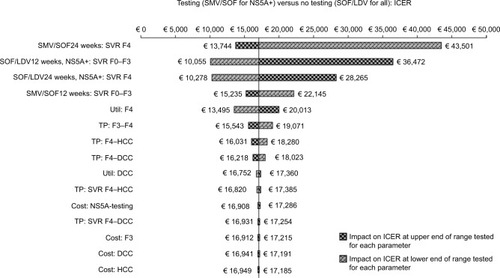
Figure 2 Probabilistic sensitivity analysis: two-way cost-effectiveness acceptability curve.
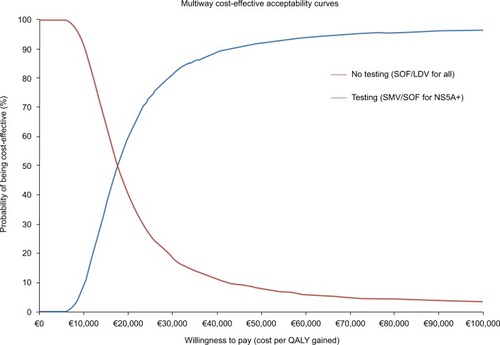
Table 6 Results of scenario analyses
