Figures & data
Table 1 Unit costs included in the analysis
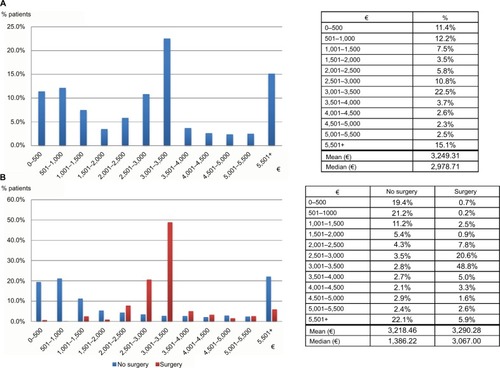
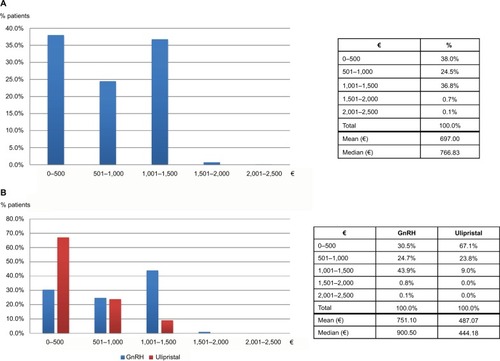
Table 2 Demographics and health care history of women with diagnosis of uterine fibroids: comparison between surgical and nonsurgical patients
Figure 1 Number of patients, included in the main study, divided according to the following criteria: health districts in nonsurgical patients (A), age groups in nonsurgical patients (B), type of surgery in surgical patients (C), and age groups in surgical patients (D).
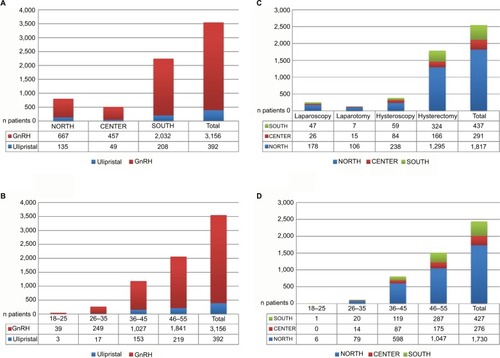
Table 3 Demographics and health care history of women with diagnosis of uterine fibroids treated with GnRH analogs or ulipristal acetate
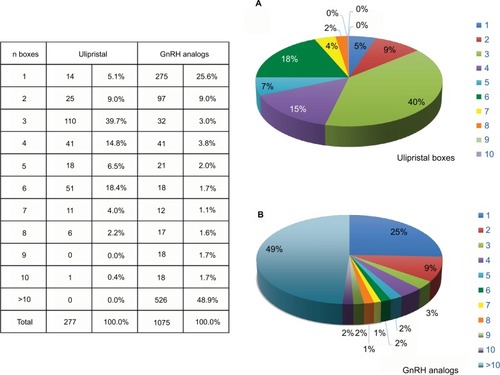
Figure 2 Number of prescribed and reimbursed medication packages per patient. Ulipristal (A) and GnRH analogs (B).