Figures & data
Figure 1 Study design.
Abbreviations: AE, adverse event; GCA, giant cell arteritis; OGC, oral glucocorticoid.
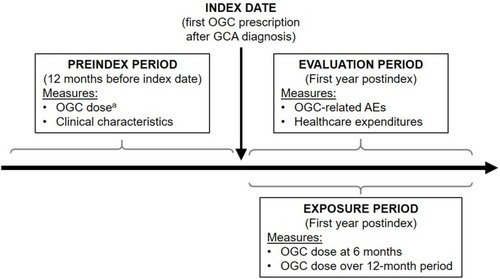
Figure 2 Sample attrition.
Abbreviations: aTNF, anti-tumor necrosis factor inhibitor; CTA, computed tomography angiography; GCA, giant cell arteritis; MRA, magnetic resonance angiography; OGC, oral glucocorticoid; PET-CT, positron emission topography-computed tomography; TAB, temporal artery biopsy; TCZ, tocilizumab.
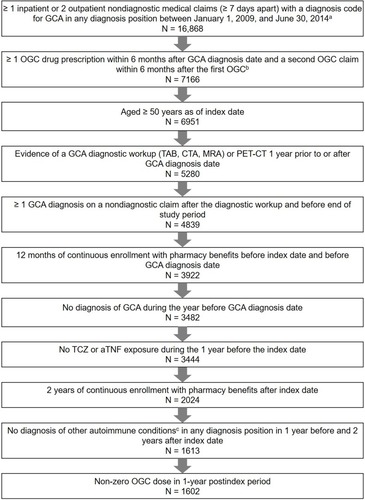
Table 1 Preindex Demographics and Clinical Characteristics
Figure 3 Proportion of patients with AEs and mean (unadjusted) AE-related costs (USD) in the 1-year postindex period.
Abbreviations: AE, adverse event; OGC, oral glucocorticoid.
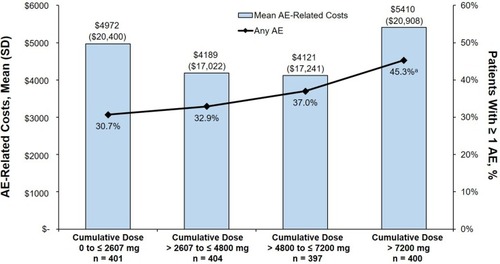
Figure 4 Predicted mean and predicted median AE-related costs (USD) in the 1-year postindex period.
Abbreviations: AE, adverse event; OGC, oral glucocorticoid.
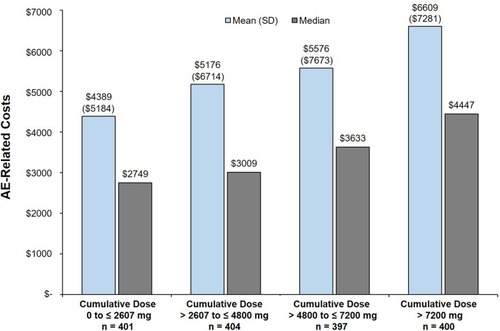
Table 2 Model Output from Gamma Cost Models
Data Availability
Qualified researchers may request access to data through the clinical study data request platform, www.clinicalstudydatarequest.com. Further details on Roche’s criteria for eligible studies are available here (https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Roche.aspx). For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
