Figures & data
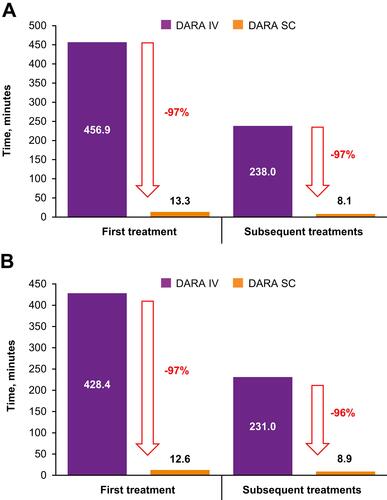
Figure 1 Median active HCP time for first and subsequent treatments (A) primary analysis and (B) sensitivity analysis*.
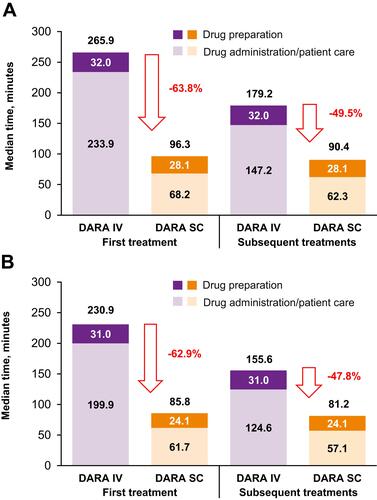
Figure 2 Duration of drug administration and active HCP involvement for first and subsequent treatments (A) primary analysis and (B) sensitivity analysis*.
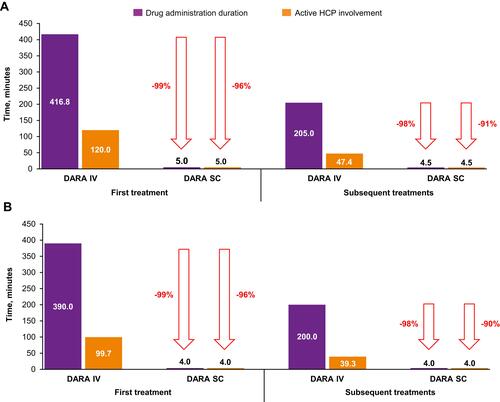
Figure 3 Median active HCP time: pre-specified activities in patient care area.
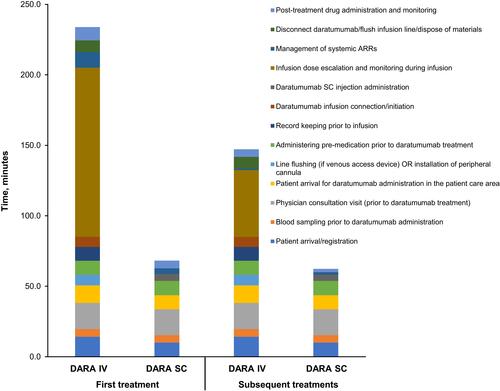
Figure 4 Estimated active HCP time per patient per year (pharmacy/drug preparation area and patient care area combined).
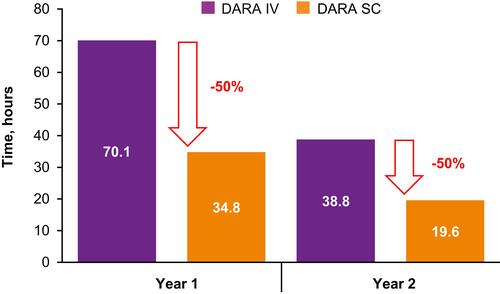
Figure 5 Estimated chair time for first and subsequent treatments (A) primary analysis and (B) sensitivity analysis*.