Figures & data
Table 1 Regular Dosages for Drugs Administered in Patients with UC
Figure 1 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for publication screening and selection. aEMBASE + Medline.
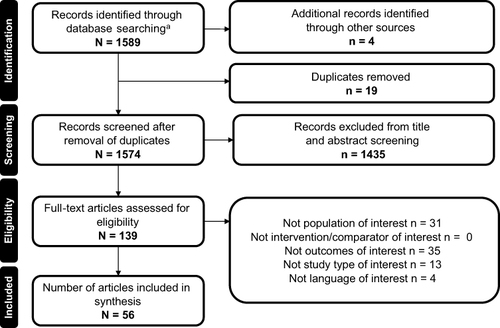
Figure 2 Proportion of patients treated with dose escalation due to loss of responseCitation31,Citation44,Citation46,Citation63,Citation66–68,Citation75,Citation76 or any reason.Citation22–29,Citation32,Citation34,Citation35,Citation37,Citation38,Citation40–42,Citation45,Citation47–52,Citation55,Citation57–62,Citation64,Citation65,Citation69,Citation71,Citation72,Citation74 Studies originating in multiple countries and including multiple anti-TNF therapies were counted more than once, giving a total number of references higher than the 48 publications found.
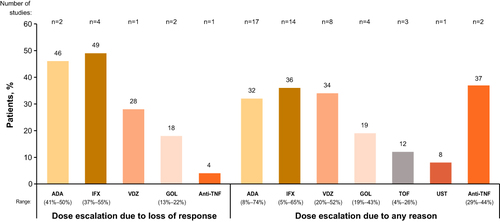
Figure 3 (A) Rates of clinical responseCitation31,Citation32,Citation40,Citation44,Citation55,Citation61,Citation62,Citation66–69 and (B) clinical remissionCitation31,Citation32,Citation34,Citation55,Citation59,Citation61,Citation62,Citation66,Citation67,Citation69,Citation75 after dose escalation. a20% of patients were in clinical remission at time of dose escalation. bPartial remission; only 20 observed cases for partial remission at 12 months (ie, data missing for 21 of the original 41 patients). c10% of patients were in clinical remission at the time of dose escalation, with such patients who violated protocol being treated with dose escalation at the discretion of the treating physician. dData reported for a subpopulation of patients treated with dose escalation (n=20/40) for whom 16-week follow-up data were available.
Table 2 Clinical Remission and Clinical Response Following Treatment Switch
