Figures & data
Table 1 Baseline Demographics and Clinical Characteristics
Figure 1 Identification of the study population.
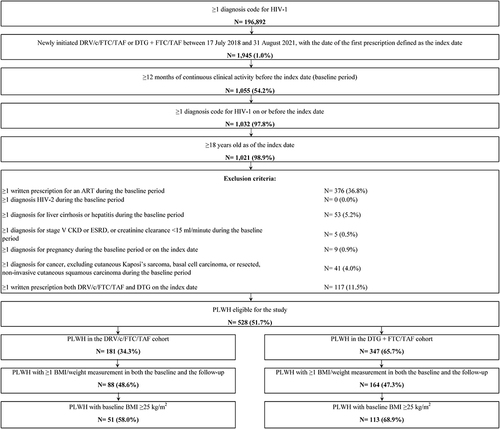
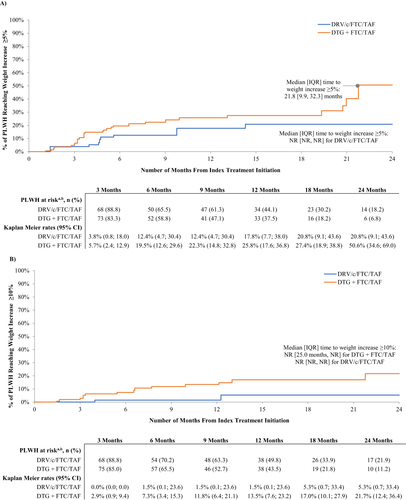
Figure 2 Kaplan Meier curves of time to weight/BMI increase above threshold. (a) Time to weight increase ≥5%. (b) Time to weight increase ≥10%. (c) Time to BMI increase ≥5%. (d) Time to BMI increase ≥10%.
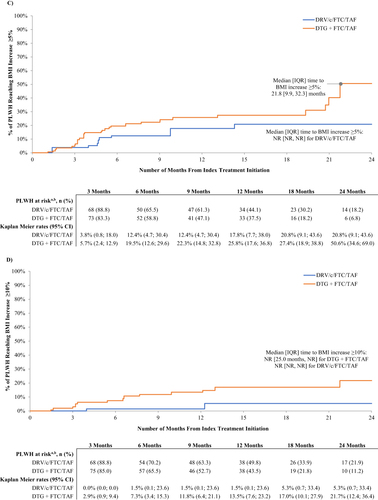
Figure 3 Comparison of time to weight or BMI increase outcome.

Table 2 Baseline BMI Category and Proportion of PLWH with BMI Category Shiftsa During Follow-Up