Figures & data
Figure 1 Sample selection diagram.
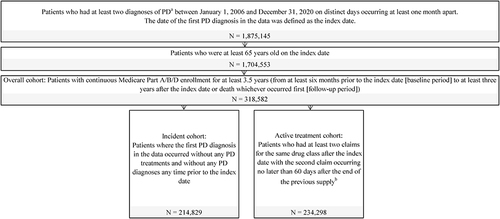
Table 1 Baseline Characteristics Stratified by Patient Cohort
Figure 2 Treatment patterns of incident cohort. (A) Distribution of first, second, and third lines of therapy; (B) levodopa dosing; (C) time to treatment initiation; (D) time to treatment discontinuation.
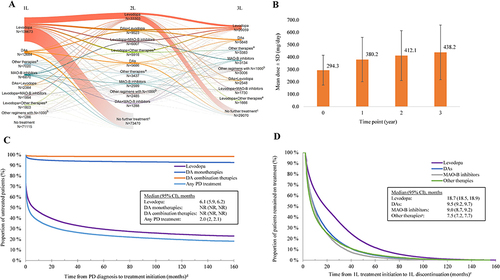
Table 2 All-Cause HRU Over 6-Month Cycles Associated with PD Symptoms Among Overall Cohort
Table 3 All-Cause HRU Over 6-Month Cycles Associated with Drug-Specific AEs Among Active Treatment Cohort
Table 4 All-Cause Healthcare Costs Over 6-Month Cycles Associated with PD Symptoms Among Overall Cohort
Table 5 All-Cause Healthcare Costs Over 6-Month Cycles Associated with Drug-Specific AEs Among Active Treatment Cohort
