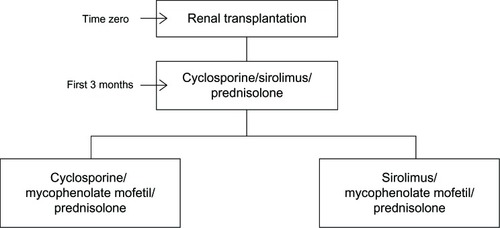
Figures & data
Table 1 Model inputs and data sources for the budget impact analysis
Table 2 Cost of immunosuppressive agents in SRL versus CsA based therapies in Iran (2011–2012)
Table 3 Cost of adverse events per patient in renal transplantation therapy in Iran (2011–2012)
Table 4 Total adverse events cost related to SRL versus CsA based therapies in renal transplantation therapy in Iran (2011–2012)
Figure 2 Budget impact of using SRL to replace the current conventional therapy with CsA in Iran (2011–2012).
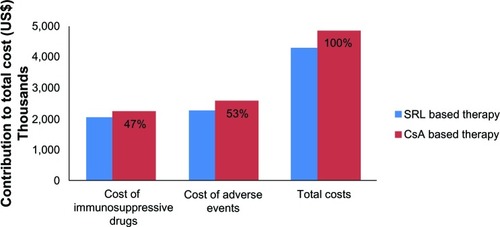
Table 5 Budget impact results of conversion from CsA to SRL in renal transplantation therapy for insurance organizations in Iran (2011–2012)
Figure 3 Sensitivity analyses results for SRL market price in Iran (2011–2012). At a price between $1.22 and $1.31, the budget difference would be zero.
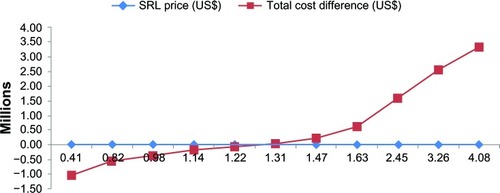
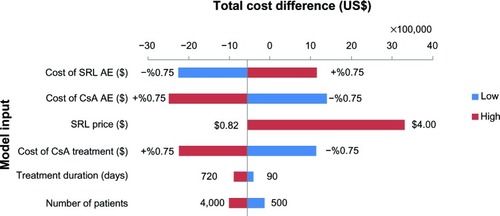
Figure 4 Sensitivity analyses: total BI difference between SRL and CsA based therapies (2011–2012).
Abbreviations: AE, adverse events; BI, budget impact; CsA, cyclosporine a; SRL, sirolimus.