Figures & data
Table 1 Demographics of the Study Population
Table 2 Compliance
Table 3 Joint Range of Motion (ROM)
Figure 1 Change in joint mobility.
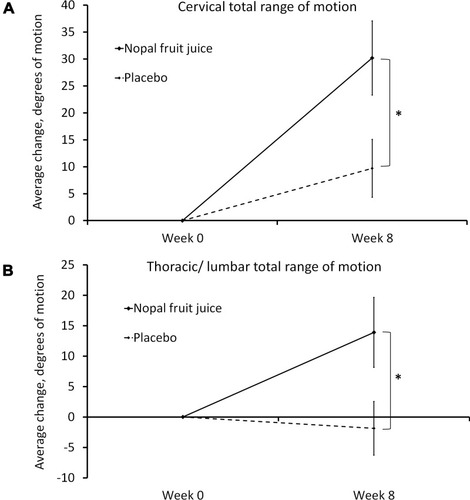
Figure 2 Change in pain interfering with physical functioning.
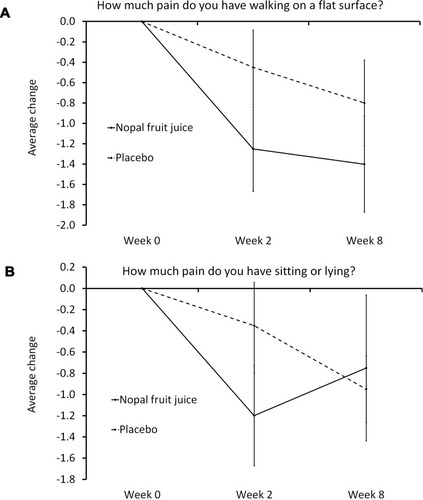
Figure 3 Daily activities.
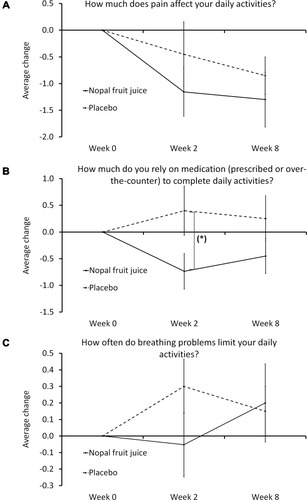
Table 4 Serum Cytokine Levels (Pg/mL)
Figure 4 Serum Eotaxin levels.
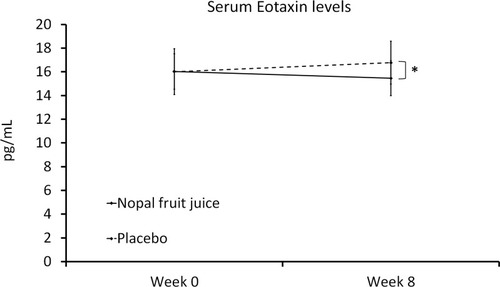
Table 5 Serum C-Reactive Protein Levels (Mg/L)
