Figures & data
Table 1 Comparison of Demographic and Basal Clinical Characteristics of Patients Between XueBiJing Injection and Placebo Groups
Table 2 Rate of Patients with Acute Respiratory Distress Syndrome and Septic Shock, The Baseline Settings of Mechanical Ventilation, and the Frequency of Antimicrobial Prescriptions for XueBiJing Group vs Placebo Group Using Descriptive Statistics for the Intention-to-treat Populations
Table 3 The Primary and Two Secondary Outcomes
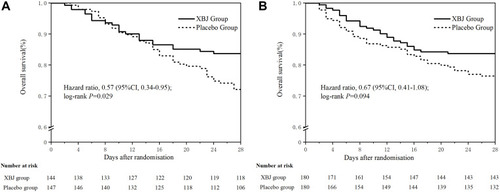
Figure 1 Kaplan–Meier survival curve of overall survival after XueBiJing (XBJ) and placebo for patients in (A) elderly group: patients with XBJ had a significantly inferior overall survival to those who with placebo. The HR of was 0.57 (95%CI, 0.34–0.95; P=0 0.029), (B) nonelderly group: the HR was 0.67 (95%CI, 0.41–1.08; P=0 0.0949).