Figures & data
Table 1 Baseline demographics of patients
Table 2 Clinical characteristics of patients
Table 3 Incidence of treatment-emergent skin or subcutaneous tissue and gastrointestinal adverse events affecting ≥1% of patients
Figure 1 Incidence of dry skin among patients in the TDiclo, placebo, and control groups.
Abbreviation: TDiclo, diclofenac sodium topical solution.
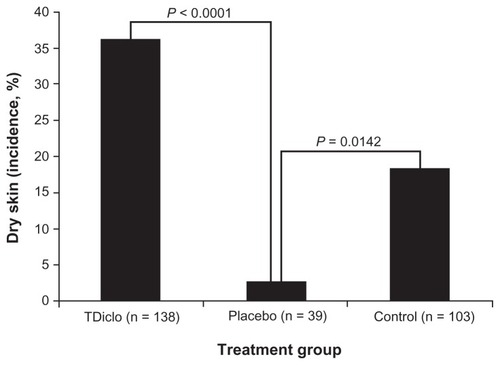
Table 4 Overall incidence of treatment-emergent skin or subcutaneous tissue and gastrointestinal adverse events that resulted in study discontinuation in ≥1% of patients
Figure 2 Incidence of application site-related adverse events among patients in the TDiclo, placebo, and control groups.
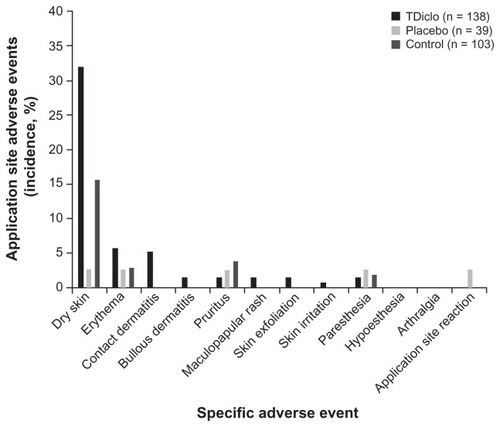
Table 5 Changes in blood pressure and key laboratory measurements from baseline to the final visit