Figures & data
Table 1 Summary of the main central and peripheral actions of leptin
Figure 1 (A) Effects of leptin production in a young state. White adipocytes, mostly subcutaneous, secrete normal levels of leptin. Peripherally, leptin contributes to insulin sensitivity and free fatty acids oxidation in liver, muscle, and adipose tissue. Centrally, leptin reach its targets through its transport across the blood brain barrier provided by an active saturable transport system. Leptin is also transported through the circumventricular organs. Its binding to LEPRs expressed in the arcuate nucleus of the hypothalamus leads to an increase of POMC and a decrease of NPY/AgRP levels. This modulation of specific neuronal populations triggers SNS activation, which leads to an increase of UCP1 transcription and thermogenesis in BAT. (B) Effects of leptin production in middle-age condition. Subcutaneous fat begins to be redistributed and white adipocytes, mostly visceral, produce a high amount of leptin. Peripherally, leptin resistance develops in the liver, muscle, and adipose tissue and causes a decrease in insulin sensitivity and FFAs oxidation and an increase in lipolysis. Centrally, alterations in the blood brain barrier decrease leptin transport to the CNS, which leads to a reduction in the production of POMC. This diminution blunts SNS signaling and induces BAT atrophy and leads to a decrease in both UCP1 levels and thermogenesis. This BAT atrophy also contributes to an increase in leptin secretion. (C) Effects of old age on leptin secretion. The subcutaneous depot is atrophied and fat accumulates viscerally and mostly in ectopic depots. High levels of leptin are secreted by visceral adipose tissue, concomitantly with an increase in glucose intolerance peripherally probably attributed to a loss of leptin signaling. Centrally, levels of POMC are still decreased, which leads to a more important atrophy of BAT and quiescent thermogenesis. Since BAT is inactive, levels of secreted leptin by this tissue are increased.
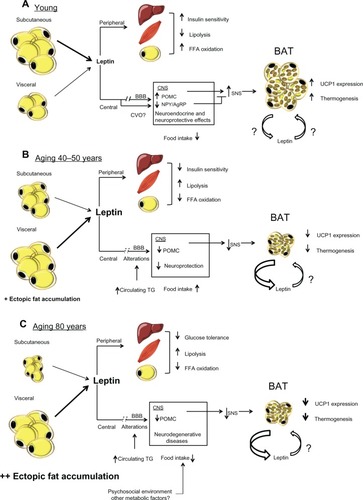
Figure 2 Representation of the different modifications regarding the redistribution of body fat, body weight, leptin resistance, and circulating levels of leptin, adiponectin, and insulin in conditions of youth, middle age, healthy aging, and unhealthy aging. Red and grey represent visceral and subcutaneous adipose tissue, respectively.
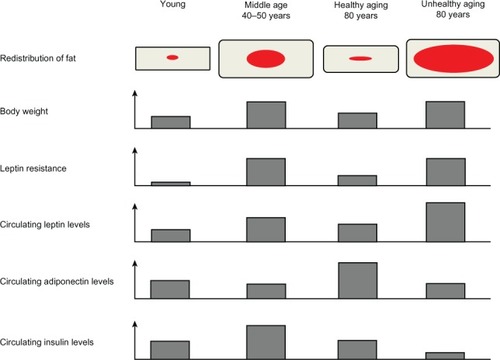
Table 2 Modifications observed in different adipose tissue depot during aging