Figures & data
Figure 1 Symptom progression in Alzheimer’s disease.
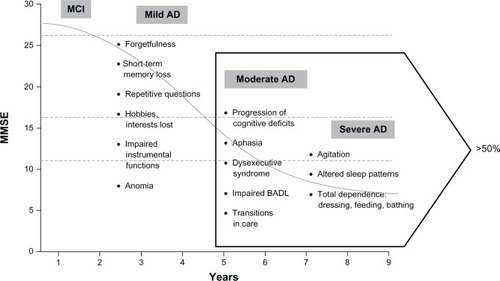
Table 1 Language impairments in Alzheimer’s dementia
Figure 2 Effect of donepezil 23 mg/day on language function after 24 weeks of treatment in patients with moderate to severe Alzheimer’s disease. Mean change in LS from baseline to week 24 in SIB-L scores (A) and 21-item SIB-derived language subscale scores (B).
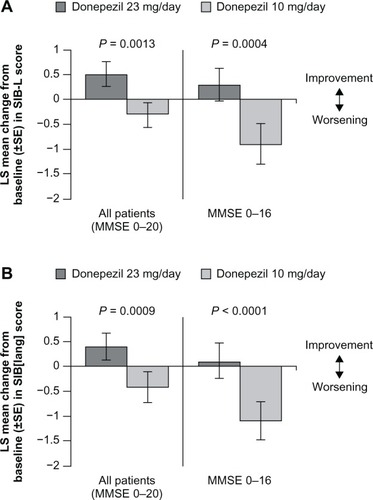
Figure 3 Effect of rivastigmine on language function after 26 weeks of treatment in patients with mild to moderate Alzheimer’s disease.
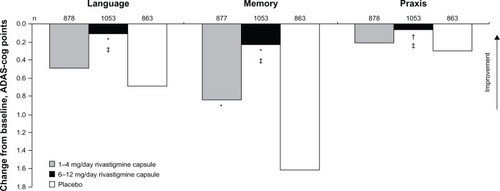
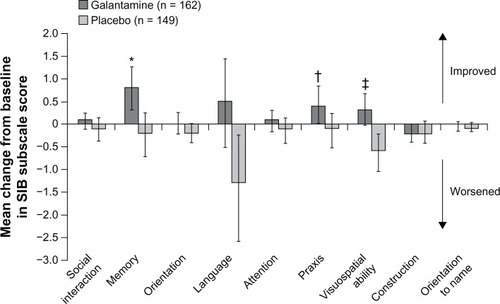
Figure 4 Effect of galantamine on language function after 26 weeks of treatment in patients with severe Alzheimer’s disease.