Figures & data
Table 1 Baseline Characteristics
Table 2 Efficacy and Safety of Rivaroxaban Classified by Multiplex Dosage Regimen
Figure 2 Pattern of rivaroxaban dose associated safety observation. (A) Cases of hemorrhages in different dose gradients without loading. (B) Percentage of hemorrhages in different dose gradients without loading. (C) Cases of hemorrhages in different dose gradients with loading. (D) Percentage of hemorrhages in different dose gradients without loading. (E) Cases of hemorrhages in different loading conditions. (F) Percentage of hemorrhages in different loading conditions.
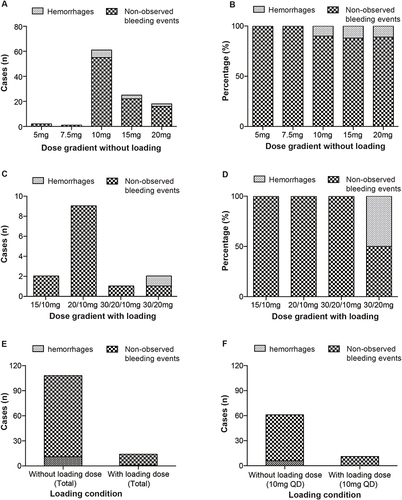
Figure 3 Pattern of rivaroxaban dose associated efficacy observation. (A) Cases of thrombus persisting in different dose gradients without loading. (B) Percentage of thrombus persisting in different dose gradients without loading. (C) Cases of thrombus persisting in different dose gradients with loading. (D) Percentage of thrombus persisting in different dose gradients with loading. (E) Cases of thrombus persisting in different loading conditions. (F) Percentage of thrombus persisting in different loading conditions.
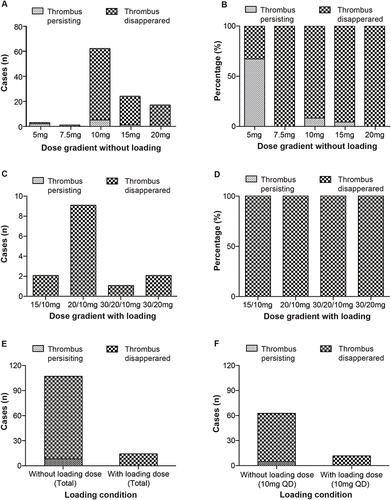
Figure 4 Pattern of complication or comorbidity associated safety observation. (A) Cases of hemorrhages in patients with and without malignancy. (B) Cases of hemorrhages in 10mg rivaroxaban recipients with and without malignancy. (C) Cases of hemorrhages in patients with and without infection. (D) Cases of hemorrhages in 10mg rivaroxaban recipients with and without infection. (E) Cases of hemorrhages in patients with different eGFR levels. (F) Cases of hemorrhages in 10mg rivaroxaban recipients with different eGFR levels. Note: eGFR = glomerular filtration rate estimated using the Cockcroft–Gault formula.
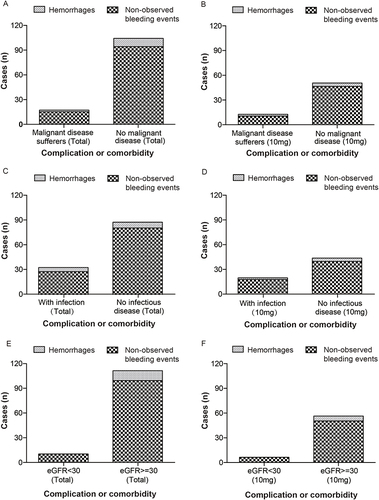
Figure 5 Pattern of complication or comorbidity associated efficacy observation. (A) Cases of thrombus persisting in patients with and without malignancy. (B) Cases of thrombus persisting in 10mg rivaroxaban recipients with and without malignancy. (C) Cases of thrombus persisting in patients with and without infection. (D) Cases of thrombus persisting in 10mg rivaroxaban recipients with and without infection. (E) Cases of thrombus persisting in patients with different eGFR levels. (F) Cases of thrombus persisting in 10mg rivaroxaban recipients with different eGFR levels. Note: eGFR = glomerular filtration rate estimated using the Cockcroft–Gault formula.
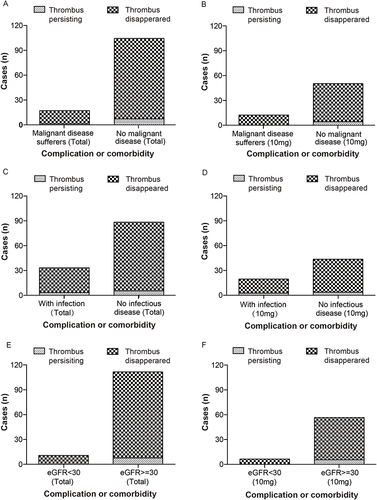
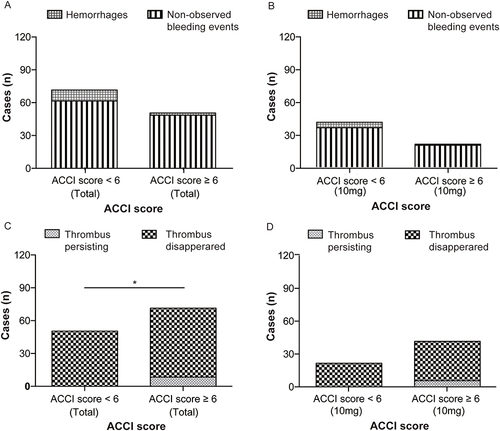
Figure 6 Efficacy and safety outcomes of patients with different ACCI scores. (A) Cases of hemorrhages in patients with different ACCI scores. (B) Cases of hemorrhages in 10mg rivaroxaban recipients with different ACCI scores. (C) Cases of thrombus persisting in patients with different ACCI scores. (D) Cases of hemorrhages in 10mg rivaroxaban recipients with different ACCI scores. Note: ACCI= age-adjusted Charlson comorbidity index. *P<0.05.
Data Sharing Statement
The raw data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study. Data are, however, available from the authors upon reasonable request and with permission of the corresponding author (Wangshu Dai).